Professional Documents
Culture Documents
WPT Iit Jee
WPT Iit Jee
Uploaded by
Deena chemist0 ratings0% found this document useful (0 votes)
6 views2 pagesOriginal Title
WPT IIT JEE
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
6 views2 pagesWPT Iit Jee
WPT Iit Jee
Uploaded by
Deena chemistCopyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
You are on page 1of 2
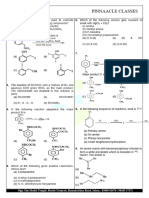
PINNAACLE CLASSES
46. Ans. 7
Sol. wt. of KOH in mixture = 2.80
wt. of Ca(OH)2 in mixture = 3.33
2. 8 3 . 33×2
+
So 56 74 = V × 10–3 × 20
0.14 = V × 2 × 10–2
14
V= 2 mL = 7 ml
47. Ans. 6
Sol. Zn2+ + H2O Zn(OH)+ + H+
c (1 – ) c c
cα2
KH = 10–9 = (1−α )
Considering <<1
= √ 10 = 10–3
−6
[H+] = c = 10–6 or pH = 6
48. 6 JEE Main 2020
9.8 −3
MH S O4 ⇒=10
2
98× 100
4 −3
M NaOH ⇒ =10
40 ×100
−3 −3
40 ×10 −10 ×10 ×2 20 −3
¿ = × 10
50 50
¿
2 −3
pOH = ×10
5
pOH =3.397
pH=10.603
49. 141 JEE Main 2021
−9
K SP ( PbI 2) =8 ×10
+2 −¿ ( aq ) ¿
PbI 2 ( s ) ⇌ Pb ( aq )+2 I
S+0.1 2S
K SP =[ Pb+2 ] ¿ ¿
−9 2 −9 2
8 ×10 =( S+ 0.1 )( 2 S ) ⇒ 8 ×10 ≃ 0.1 × 4 S
2 −8
⇒ S =2× 10
S=1.414 ×10 mol/Lit
−4
¿ x × 10 mol/Lit ∴ x=141.4 ≃ 141
−6
50. 7 JEE Main 2021
Opp. Om Shakti Temple, Beside Naturals, Ramakrishna Road, Salem : 83009 81676 / 98403 37371
PINNAACLE CLASSES
3 −¿¿
+¿+P O 4 ¿
( N H 4 ) 3 P O4 ⇌ 3 NH 4
¿
1
pH= p k a+
2
{ p k w − p k a −p k b }
1
pH=5.23+ {14−5.23−4.75 }
2
1
pH=5.23+ ( 4.02 )=7.24 (Nearest Integer)
2
Opp. Om Shakti Temple, Beside Naturals, Ramakrishna Road, Salem : 83009 81676 / 98403 37371
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5814)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1092)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (845)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (590)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (897)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (540)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (348)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (822)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (401)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- DPT 31 Xii Centre Rasi Che Neet Key 07-12-23Document8 pagesDPT 31 Xii Centre Rasi Che Neet Key 07-12-23Deena chemistNo ratings yet
- WPT Xi Centre Che Neet Key 10-12-23Document3 pagesWPT Xi Centre Che Neet Key 10-12-23Deena chemistNo ratings yet
- Xii DPT Bot 29.03.24Document6 pagesXii DPT Bot 29.03.24Deena chemistNo ratings yet
- DPT 31 Xii Centre Rasi Che Iit Key 07-12-23Document4 pagesDPT 31 Xii Centre Rasi Che Iit Key 07-12-23Deena chemistNo ratings yet
- DPT 31 Xii Centre Rasi Che Iit 07-12-23Document4 pagesDPT 31 Xii Centre Rasi Che Iit 07-12-23Deena chemistNo ratings yet
- Revision Schedule 23-24Document22 pagesRevision Schedule 23-24Deena chemistNo ratings yet
- Electrochemistry 45 KeyDocument10 pagesElectrochemistry 45 KeyDeena chemistNo ratings yet
- WPT Xi Rasi Che Neet Key 2-12-23Document2 pagesWPT Xi Rasi Che Neet Key 2-12-23Deena chemistNo ratings yet
- Coordination WSDocument3 pagesCoordination WSDeena chemistNo ratings yet
- Xi Rasi Phy Iit WPT 19.02.24 KeyDocument1 pageXi Rasi Phy Iit WPT 19.02.24 KeyDeena chemistNo ratings yet
- DPT 33 Centre Rasi Iit Jee Che Key 09-12-23Document4 pagesDPT 33 Centre Rasi Iit Jee Che Key 09-12-23Deena chemistNo ratings yet
- LT DPT Jee Key 22.02.24Document1 pageLT DPT Jee Key 22.02.24Deena chemistNo ratings yet
- Rasi WPT Xi Che Iit Key 01-1-1-24Document2 pagesRasi WPT Xi Che Iit Key 01-1-1-24Deena chemistNo ratings yet
- WPT CRP Xi Che Neet Key 18-02-24Document6 pagesWPT CRP Xi Che Neet Key 18-02-24Deena chemistNo ratings yet
- LT DPT 15 Jee 21.02.2024 KeyDocument1 pageLT DPT 15 Jee 21.02.2024 KeyDeena chemistNo ratings yet
- LT RPT Jee Phy 18.02.24Document4 pagesLT RPT Jee Phy 18.02.24Deena chemistNo ratings yet
- LT RPT2 Jee Che 18-02-24Document2 pagesLT RPT2 Jee Che 18-02-24Deena chemistNo ratings yet
- WPT Xi Centre Che Neet Key 21-11-23Document4 pagesWPT Xi Centre Che Neet Key 21-11-23Deena chemistNo ratings yet
- LT Jee DPT 15.02.24Document3 pagesLT Jee DPT 15.02.24Deena chemistNo ratings yet
- Xi ND Phy Iit CPT 19.02.24Document4 pagesXi ND Phy Iit CPT 19.02.24Deena chemistNo ratings yet
- Xi ND CPT ZoologyDocument4 pagesXi ND CPT ZoologyDeena chemistNo ratings yet
- X ND WPT Che 1 17-10-22Document1 pageX ND WPT Che 1 17-10-22Deena chemistNo ratings yet
- F BlockDocument10 pagesF BlockDeena chemistNo ratings yet
- CPT Rasi Xi Che NeetDocument5 pagesCPT Rasi Xi Che NeetDeena chemistNo ratings yet
- Dptchem & Zoo01.2024Document2 pagesDptchem & Zoo01.2024Deena chemistNo ratings yet
- Jee GrandDocument16 pagesJee GrandDeena chemistNo ratings yet
- Xi Rasi Neet Che WPT QP 22.01.2024Document3 pagesXi Rasi Neet Che WPT QP 22.01.2024Deena chemistNo ratings yet
- Xi CRP Neet Che WPT QP 31.12.2023Document3 pagesXi CRP Neet Che WPT QP 31.12.2023Deena chemistNo ratings yet