Professional Documents
Culture Documents
BIOENERGETICS Notes 4
Uploaded by
Hya Laniog0 ratings0% found this document useful (0 votes)
17 views3 pagesOriginal Title
BIOENERGETICS notes 4
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
17 views3 pagesBIOENERGETICS Notes 4
Uploaded by
Hya LaniogCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 3
Enzymes
A catalyst that accelerates the forward
and reverses rates of chemical reactions
within cells without being consumed or
changed in the reaction.
Reactions are catalyzed by a specific
enzyme.
Make contact at precise locations on the
surface of cell structures.
Enzymes do not operate at the same rate
- While one enzyme is activated, the
other enzymes around it are
deactivated until its reaction is
complete.
Naming: 2. The enzyme catalyzes the chemical
reaction
Suffix - ase
Each enzyme has an optimum pH range.
Hydrolase - adds water during hydrolysis Changing the pH outside of this range will
reactions slow enzyme activity.
Protease - interacts with protein
Oxydase - adds oxygen to a substance
Ribonuclease - splits apart RNA
The Lock and Key Mechanism of
Enzymes
Enzymes become activated when its active
site is joined in “perfect fit” with the active
site on the substrate.
Interaction steps:
1. The active site of the enzyme 3. An end product is produced and
achieves a perfect fit with the active the enzyme is regenerated
site of the substrate to form an unchanged.
enzyme-substrate complex.
such as in the growth and repair of tissues.
Catabolic reactions - breakdown of
substances (decomposition).
Anabolism and Catabolism
Induced Fit Model of Enzymes
The enzyme is able to slightly change its
shape and best fit the reactants as it
attaches.
Lock and Key and Induced Fit Mechanism
allow/facilitate the reactants to orient
toward each other so that the chemical
reaction can occur easily (faster). Reactants → Products
Temperature and pH changes affect the Synthesis (Anabolic) reactions include:
enzyme structure and function.
- Amino acids → Protein molecules
Cofactors - ADP + Pi → ATP + H2O
- Non-protein substances enzymes need to - Formation of carbohydrates and
function properly. Ex: Mg, Zn lipids
- Normally part of the enzyme’s active part
to make it functional. Decomposition reactions
Coenzymes
- Organic cofactors such as vitamins. Products → Reactants
- Glycogenolysis
Chemical Reactions - Lipolysis
- ATP → ADP + Pi
Interactions of atoms, ions, molecules, and - Hydrolysis > water is needed to
compounds. break a molecule/substance.
Metabolism - all reactions in the body.
Hydrolysis
Anabolic reactions - synthesis reactions
corresponding gain in valence (or
Complex organic molecules such as positive charge).
carbohydrates, lipids, and proteins are
degraded, or catabolized into simpler Reduction
forms that the body can easily use. - Gain of electrons, loss of valence.
Energy is released when chemical bonds
are broken by the addition of the
constituents of water (H+ and OH-).
AB + HOH → A-H + B-OH
*Hydrolysis of each reaction is catalyzed by
an enzyme.
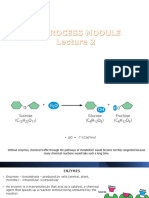
Examples of hydrolysis reactions in the
body:
- Hydrolysis of disaccharides:
enzyme-sucrase
Sucrose → glucose + fructose
- Hydrolysis of lipid: enzyme - lipase
Lipids → fatty acid + glycerol backbone
- Digestion of proteins: enzyme - protease
Dipeptide → amino acid + amino acid
Reversible reactions
At equilibrium: reactant formation rate =
production formation rate
Example:
Bicarbonate buffer system - maintains a
constant level of H+ for proper
physiological processes.
H+ + HCO3- < —-- > H2CO3
Oxidation and Reduction Reactions
Reaction that involve the transfer of
oxygen atoms, hydrogen atoms, or
electrons.
Oxidation
- There is a loss of electrons with a
You might also like
- Module 6 in BiochemistryDocument19 pagesModule 6 in BiochemistryjeromeNo ratings yet
- Butadien Production Scribd File PDFDocument22 pagesButadien Production Scribd File PDFasharab70100% (2)
- Enzymes: Dr. Walid Said Zaki Lecturer of Biochemistry & Molecular BiologyDocument52 pagesEnzymes: Dr. Walid Said Zaki Lecturer of Biochemistry & Molecular Biologyeman el saeedNo ratings yet
- What Are Enzymes?: EnzymeDocument4 pagesWhat Are Enzymes?: EnzymeMark Jayson ContrerasNo ratings yet
- Enzyme ComponentsDocument36 pagesEnzyme ComponentsJanine CambaNo ratings yet
- Biochemistry NCMA113 Midterm Notes PDocument3 pagesBiochemistry NCMA113 Midterm Notes PVaishnavi LoganathanNo ratings yet
- ENZYMES (Autosaved)Document12 pagesENZYMES (Autosaved)zaynab.z.sidNo ratings yet
- Prelab 4Document6 pagesPrelab 4Trần Xuân QuỳnhNo ratings yet
- Enzymes: - Sucrase - Lactase - MaltaseDocument4 pagesEnzymes: - Sucrase - Lactase - MaltaseKate Nicole EjercitoNo ratings yet
- Biological Molecules: Enzymes and Their FunctionsDocument50 pagesBiological Molecules: Enzymes and Their FunctionsBernard D. Fajardo Jr.100% (1)
- Enzymes: by Nura Malahayati THP-FP UnsriDocument71 pagesEnzymes: by Nura Malahayati THP-FP UnsriIkhsan muttaqinNo ratings yet
- Chapter 4 Enzymes and VitaminsDocument9 pagesChapter 4 Enzymes and Vitaminsvictoria cablayNo ratings yet
- CC-LEC-NOTES-1Document4 pagesCC-LEC-NOTES-1CDNo ratings yet
- Chem113 LecDocument15 pagesChem113 LecLindon TibleNo ratings yet
- Enzymes: Enzymes Homeostasis Activation EnergyDocument6 pagesEnzymes: Enzymes Homeostasis Activation EnergyGowthami MarreddyNo ratings yet
- Pre-Lab 3: 1. What Is A Catalyst and A Catalysis Reaction?Document3 pagesPre-Lab 3: 1. What Is A Catalyst and A Catalysis Reaction?Trần NguyênNo ratings yet
- Microbial Metabolism and Energy ProductionDocument40 pagesMicrobial Metabolism and Energy ProductionVzilNo ratings yet
- OUTLINE8Document8 pagesOUTLINE8Hjm ArNo ratings yet
- Biological Molecules EnzymesDocument50 pagesBiological Molecules EnzymesFrance Angel AlbertoNo ratings yet
- EnzymesDocument7 pagesEnzymesSheena Grace B. LobatonNo ratings yet
- EnzymesDocument46 pagesEnzymesHighlifeNo ratings yet
- EnzymesDocument22 pagesEnzymeslovelykissNo ratings yet
- 1.3 Cell BioDocument7 pages1.3 Cell BioAbz GindiNo ratings yet
- 2.1.4 Biology Ocr ADocument5 pages2.1.4 Biology Ocr AFatima AfifiNo ratings yet
- EnzymeDocument11 pagesEnzymeprashant kumarNo ratings yet
- Enzymes - BiochemistryDocument40 pagesEnzymes - Biochemistrysunil patelNo ratings yet
- Biology 2nd QuarterDocument26 pagesBiology 2nd QuarterCosmo FiendNo ratings yet
- CH 2306 Lecture Notes Part 2 Enzymology-1Document5 pagesCH 2306 Lecture Notes Part 2 Enzymology-1owegibrian479No ratings yet
- Chapter 3 Enzymes Hormones VitaminsDocument133 pagesChapter 3 Enzymes Hormones VitaminsTran Danh NhanNo ratings yet
- Enzymes - Lecture NotesDocument8 pagesEnzymes - Lecture NotesssekitoolekohakimNo ratings yet
- Enzyme NoteDocument4 pagesEnzyme NoteYolanda JesslinaNo ratings yet
- Biochem NotesDocument52 pagesBiochem NotesAnonymous 4Ib1ByFC100% (2)
- ENZYMES SPEED UP REACTIONSDocument20 pagesENZYMES SPEED UP REACTIONSKoushik KanjilalNo ratings yet
- Enzymes PDFDocument36 pagesEnzymes PDFAltamashNo ratings yet
- Enzymes Lower Activation Energy for Chemical ReactionsDocument3 pagesEnzymes Lower Activation Energy for Chemical ReactionsdetNo ratings yet
- O Level Biology Notes - EnzymesDocument3 pagesO Level Biology Notes - Enzymesdet100% (1)
- EnzymeDocument3 pagesEnzymedetNo ratings yet
- 3.5 Enzymes 2Document64 pages3.5 Enzymes 2Alondra SagarioNo ratings yet
- Bio-Organic Chemistry (Unit-V)Document32 pagesBio-Organic Chemistry (Unit-V)PG ChemistryNo ratings yet
- Chapter 2 - MetabolismDocument27 pagesChapter 2 - MetabolismKevin -No ratings yet
- Chapter 2: Metabolism: By: 12 - Mia - 03Document27 pagesChapter 2: Metabolism: By: 12 - Mia - 03Kevin -No ratings yet
- Cell Bio Chapter 6Document32 pagesCell Bio Chapter 6GuteNo ratings yet
- Enzymes. RagaviDocument63 pagesEnzymes. Ragavikavi bharathiNo ratings yet
- Enzymes ...Document13 pagesEnzymes ...Glen MangaliNo ratings yet
- Human Body Is A Product of Different Chemical Reactions and Processes, But What Controls These Reactions?Document8 pagesHuman Body Is A Product of Different Chemical Reactions and Processes, But What Controls These Reactions?ZiarineNo ratings yet
- Bio Notes EnzymeDocument8 pagesBio Notes EnzymeMena Velante MarabeNo ratings yet
- Lecture 2Document18 pagesLecture 2warden tambiNo ratings yet
- 12 EnzDocument29 pages12 EnzMaite Aícua UbiernaNo ratings yet
- EnzymesDocument17 pagesEnzymesMartyn PereiraNo ratings yet
- Biological Chemistry: Enzyme Kinetics Part 1Document30 pagesBiological Chemistry: Enzyme Kinetics Part 1Mohammed shaffiqueNo ratings yet
- Midterm-BiochemistryDocument12 pagesMidterm-BiochemistryBiology BảoNo ratings yet
- Biochemistry: By: Angela Marie Ferrer BSN 2BDocument13 pagesBiochemistry: By: Angela Marie Ferrer BSN 2BNoemi Martinez FerrerNo ratings yet
- Enzyme kinetics and applications overviewDocument63 pagesEnzyme kinetics and applications overviewNur AishaNo ratings yet
- Introduction to Medical BiochemistryDocument115 pagesIntroduction to Medical BiochemistryfikaduNo ratings yet
- Midterm Enzymes: Classification, Specificity and Factors Affecting ActivityDocument3 pagesMidterm Enzymes: Classification, Specificity and Factors Affecting ActivityKiara Ash BeethovenNo ratings yet
- Chapter 6Document9 pagesChapter 6Georges SarkisNo ratings yet
- Biochemistry Week 3 - EnzymesDocument6 pagesBiochemistry Week 3 - EnzymesMicah JadeNo ratings yet
- Enzymes PPTDocument39 pagesEnzymes PPTsunil patelNo ratings yet
- Enzymes and Vitamins Handout and ActivityDocument24 pagesEnzymes and Vitamins Handout and ActivitycharisseNo ratings yet
- Btech Great Intro To Enzymes For MLB 104 UseDocument75 pagesBtech Great Intro To Enzymes For MLB 104 Usemaxwell amponsahNo ratings yet
- EnzymesDocument17 pagesEnzymesapi-309893409No ratings yet
- A-level Biology Revision: Cheeky Revision ShortcutsFrom EverandA-level Biology Revision: Cheeky Revision ShortcutsRating: 5 out of 5 stars5/5 (5)
- Ethylene Glycol Chemical Engineering Final Year ProjectDocument108 pagesEthylene Glycol Chemical Engineering Final Year ProjectNatarajan Girish79% (14)
- Review Methyl Chloride ProcessesDocument13 pagesReview Methyl Chloride ProcessesAlfredo PeñaNo ratings yet
- Supawadee Namuangruk: EducationDocument2 pagesSupawadee Namuangruk: EducationFadjar MulyaNo ratings yet
- Thyssenkrupp - Dehydrogenation - Star - Process STAR PDFDocument12 pagesThyssenkrupp - Dehydrogenation - Star - Process STAR PDFEmiliano RohweinNo ratings yet
- HYSYS For Ammonia Plants Using Johnson Matthey Catalysts TechnologyDocument1 pageHYSYS For Ammonia Plants Using Johnson Matthey Catalysts TechnologyAhmed Ali0% (2)
- Overflows and Gravity Drainage SystemsDocument27 pagesOverflows and Gravity Drainage SystemsDerek Huang100% (2)
- Shift Conversion OperationDocument26 pagesShift Conversion OperationMuhammad JunaidNo ratings yet
- NiCo2S4 Nanocrystals Anchored On Nitrogen-DopedDocument10 pagesNiCo2S4 Nanocrystals Anchored On Nitrogen-DopedAsmZziz OoNo ratings yet
- Phosgenation A Handbook Hal 20 EnglishDocument3 pagesPhosgenation A Handbook Hal 20 EnglishPriska Dewi AnjarsariNo ratings yet
- Us 5221800Document7 pagesUs 5221800Mochamad Abdul MalikNo ratings yet
- Kinetic Modeling and Simulation of The Selective Hydrogenation of The C - Cut of A Thermal Cracking UnitDocument8 pagesKinetic Modeling and Simulation of The Selective Hydrogenation of The C - Cut of A Thermal Cracking UnitAdrian Fernandez BelloNo ratings yet
- Atish Vishwas Supekar 5Document21 pagesAtish Vishwas Supekar 5Sudhanshu ShekharNo ratings yet
- Michaelis-Menten KineticsDocument6 pagesMichaelis-Menten Kineticscharanmann9165No ratings yet
- Heat and Mass Transfer - Modeling and SimulationDocument226 pagesHeat and Mass Transfer - Modeling and SimulationSrbislav GenicNo ratings yet
- Example Problems With Solutions PDFDocument20 pagesExample Problems With Solutions PDFsaiNo ratings yet
- USP 3112300 Isotactic Polypropylene Giulio NattaDocument11 pagesUSP 3112300 Isotactic Polypropylene Giulio NattaTrevor J. HutleyNo ratings yet
- Sorbitan Esters PlantDocument9 pagesSorbitan Esters PlantHolman SanabriaNo ratings yet
- Chapter 19 Equations of Change For Multicomponent SystemsDocument37 pagesChapter 19 Equations of Change For Multicomponent SystemsJohnNo ratings yet
- MODEL CURRICULUM FOR UNDERGRADUATE TEXTILE TECHNOLOGYDocument83 pagesMODEL CURRICULUM FOR UNDERGRADUATE TEXTILE TECHNOLOGYnivas159No ratings yet
- Green Methods For Hydrogen ProductionDocument18 pagesGreen Methods For Hydrogen ProductionSpafiu Paula Raluca100% (1)
- Chemical Engineering JournalDocument6 pagesChemical Engineering JournalRia RestianiNo ratings yet
- Catalogue of Research Groups From Moldova 2011Document102 pagesCatalogue of Research Groups From Moldova 2011aniricNo ratings yet
- Arkivoc 2018, I, AmidinasDocument44 pagesArkivoc 2018, I, AmidinasgokucharlyNo ratings yet
- Catalysts: A Review of Hydrogen Purification Technologies For Fuel Cell VehiclesDocument17 pagesCatalysts: A Review of Hydrogen Purification Technologies For Fuel Cell Vehiclesabc defgNo ratings yet
- 66 Ess16108Document9 pages66 Ess16108Jallu Pratama100% (1)
- HKDSE Chem FX ExamS5 2011 Set1 EngDocument27 pagesHKDSE Chem FX ExamS5 2011 Set1 Eng12376590No ratings yet
- The 7 Deadly Sins of Sulphur RecoveryDocument16 pagesThe 7 Deadly Sins of Sulphur RecoveryTejas AhalparaNo ratings yet
- Non-Catalytic and Heterogeneous Acid/base-Catalyzed Biodiesel Production: Recent and Future DevelopmentsDocument34 pagesNon-Catalytic and Heterogeneous Acid/base-Catalyzed Biodiesel Production: Recent and Future DevelopmentsJelian GraceNo ratings yet