Professional Documents
Culture Documents
1 A
1 A
Uploaded by
Ikfal FajarOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
1 A
1 A
Uploaded by
Ikfal FajarCopyright:
Available Formats
Rate Law Worksheet
1. The rate of a reaction is given by k [A][B]. The reactants are gases. If the volume occupied by the
reacting gases is suddenly reduced to one-fourth the original volume, what is the rate of reaction
(relative to the original rate)?
2. The following data are for Questions a through f and refer to the reaction:
A + 2B + 3C → 2Y + Z. All data were taken at 50.0°C.
trial initial [A] initial [B] initial [C] Rate of [Y]
#1 0.10 0.02 0.04 10 M/s
#2 0.10 0.03 0.04 15 M/s
#3 0.20 0.02 0.08 80 M/s
#4 0.20 0.02 0.16 160 M/s
#5 0.05 0.01 0.08 ?
a. What is the rate of formation of Y if [B] is doubled?
b. What is the rate of formation of Z in trial 3 (in M/s)?
c. What is the rate of disappearance of C in trial 2 (in M/s)?
d. What is the rate law derived for the above data?
e. What is the missing rate (trial 5) in M/s?
f. What is the rate constant?
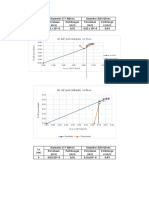
3. The times listed in the following table are those required for the concentration of S2O8-2 to
decrease by 0.00050 M as measured in an “iodine clock” reaction at 23°C. What is the rate law?
The net reaction is: S2O8-2 + 2 I- → I2 + 2 SO4-2
trial initial [S2O82-] initial [I-] Time (sec)
#1 0.0400 0.0800 39
#2 0.0400 0.0400 78
#3 0.0100 0.0800 156
#4 0.0200 0.0200 ?
a. Calculate the expected time in seconds for trial 4.
b. What is the rate law?
c. What is the rate constant?
4. Determine the rate law and calculate the rate constant for the following data.
trial initial [A] initial [B] Rate (M/s)
#1 1.00 x 10-3 0.25 x 10-3 0.26 x 10-9
#2 1.00 x 10-3 0.50 x 10-3 0.52 x 10-9
#3 1.00 x 10-3 1.00 x 10-3 1.04 x 10-9
#4 2.00 x 10-3 1.00 x 10-3 4.16 x 10-9
#5 3.00 x 10-3 1.00 x 10-3 9.36 x 10-9
#6 4.00 x 10-3 1.00 x 10-3 16.64 x 10-9
5. Determine the rate law and calculate the rate constant for the following data.
trial initial [X] initial [Y] Rate (M/s)
#1 1.00 x 10 -2
4.00 x 10 -4
6.00 x 10-3
#2 2.00 x 10 -2
4.00 x 10 -4
1.20 x 10-2
#3 4.00 x 10-2 4.00 x 10-4 2.40 x 10-2
#4 1.00 x 10 -2
8.00 x 10 -4
6.00 x 10-3
You might also like
- Mindset DweckDocument26 pagesMindset Dweckapi-357237890No ratings yet
- The Three Level Guide For Questioning Reading LiteracyDocument4 pagesThe Three Level Guide For Questioning Reading Literacyapi-3985451210% (1)
- Ipcrf 2022 2023 T1 T3Document24 pagesIpcrf 2022 2023 T1 T3Jinky Sumagaysay Joeamil100% (13)
- Math Practice Simplified: Decimals & Percents (Book H): Practicing the Concepts of Decimals and PercentagesFrom EverandMath Practice Simplified: Decimals & Percents (Book H): Practicing the Concepts of Decimals and PercentagesRating: 5 out of 5 stars5/5 (3)
- DepEd Vision, Mission and Core ValuesDocument15 pagesDepEd Vision, Mission and Core ValuesEdison Castillo GutierrezNo ratings yet
- PC35Document488 pagesPC35duker zhouNo ratings yet
- Remedial Teaching StrategiesDocument12 pagesRemedial Teaching StrategiesMarico River Conservation AssociationNo ratings yet
- Let's Practise: Maths Workbook Coursebook 6From EverandLet's Practise: Maths Workbook Coursebook 6No ratings yet
- Demo DLPDocument3 pagesDemo DLPJessemar Solante Jaron Wao100% (1)
- Media & Information Tech. Lesson 12Document2 pagesMedia & Information Tech. Lesson 12Dolly RizaldoNo ratings yet
- Characteristics of Adult LearnersDocument2 pagesCharacteristics of Adult LearnersEmelito T. ColentumNo ratings yet
- Chapter 12 - Chemical Kinetics: Answer: ADocument46 pagesChapter 12 - Chemical Kinetics: Answer: A鄭子玄No ratings yet
- Consolidation TestDocument8 pagesConsolidation TestCasper da MagnificientNo ratings yet
- Essay On The Foundations of Teaching and LearningDocument7 pagesEssay On The Foundations of Teaching and Learningapi-403689756No ratings yet
- Soal Pre Test Narrative TextDocument7 pagesSoal Pre Test Narrative TextIkfal Fajar100% (1)
- RPMS-PPST MinutesDocument2 pagesRPMS-PPST MinutesChesee Ann Sopera Dris0% (1)
- Crime Data AnalysisDocument27 pagesCrime Data AnalysisDarshan SureshNo ratings yet
- CM 1502 Lab 2Document15 pagesCM 1502 Lab 2i000years100% (1)
- Econometric FinalDocument5 pagesEconometric Finaltech damnNo ratings yet
- Chemical Kinetics: Unit 5 / Chapter 13Document24 pagesChemical Kinetics: Unit 5 / Chapter 13Aneudis Javier BritoNo ratings yet
- Chemical Kinetics DETERMINATION OF RATE LAW AND ORDER OF A REACTIONDocument2 pagesChemical Kinetics DETERMINATION OF RATE LAW AND ORDER OF A REACTIONFrancoise GurangoNo ratings yet
- Chemical Kinetics QuestionsDocument3 pagesChemical Kinetics QuestionsKlint RodneyNo ratings yet
- Chapter6solutions PDFDocument34 pagesChapter6solutions PDFBeauponte Pouky MezonlinNo ratings yet
- Lista - Atividades - 1 4 5 12Document8 pagesLista - Atividades - 1 4 5 12Brenda CarneiroNo ratings yet
- Rate Expression and Order of ReactionDocument3 pagesRate Expression and Order of ReactionMartha PrawiradirdjaNo ratings yet
- Laboratorio 1 Mecanica 1.4Document2 pagesLaboratorio 1 Mecanica 1.4Raizer BreakerNo ratings yet
- 3.2 Tests For Random Numbers: Two Types of Tests: 1. Frequency Test: UDocument12 pages3.2 Tests For Random Numbers: Two Types of Tests: 1. Frequency Test: UPradyumna A KubearNo ratings yet
- Q3 Q4 Q5 Q6 Q8 Q11Document13 pagesQ3 Q4 Q5 Q6 Q8 Q11一No ratings yet
- 209 Position vs. Time Graphs WorksheetDocument4 pages209 Position vs. Time Graphs WorksheetAnna KopsickNo ratings yet
- Pemakaian Listrik Dalam Killowat: A. Gambarlah Diagram ScatterDocument3 pagesPemakaian Listrik Dalam Killowat: A. Gambarlah Diagram ScatterEden ZaristaNo ratings yet
- Practice Problems For Instrumentation and MeasurementDocument13 pagesPractice Problems For Instrumentation and MeasurementMark lord bongatNo ratings yet
- 006 solve1DConvectionDiffusionEquationUpwindDocument5 pages006 solve1DConvectionDiffusionEquationUpwindvbkNo ratings yet
- Position vs. Time Graphs: Constant VelocityDocument4 pagesPosition vs. Time Graphs: Constant VelocityKaitlyn BernalNo ratings yet
- Método de Las Fuerzas (Formulación Matricial)Document8 pagesMétodo de Las Fuerzas (Formulación Matricial)Guillermo Carranza CiezaNo ratings yet
- M2 Mathematics Summer Vacation Homework 2022Document56 pagesM2 Mathematics Summer Vacation Homework 2022Shayan SaeedNo ratings yet
- Unit 3Document13 pagesUnit 3srik123123123No ratings yet
- Tugas No 2 Regresi LinearDocument2 pagesTugas No 2 Regresi LinearAkhmad CuyNo ratings yet
- Rcodes2 RegressionInRDocument6 pagesRcodes2 RegressionInRronNo ratings yet
- 14 Statistics SolutionDocument32 pages14 Statistics SolutionNatesh RakulNo ratings yet
- JACOB EstructuraDocument11 pagesJACOB EstructuraFreddy JoséNo ratings yet
- HW 2 CHAP 2 - YepezDocument9 pagesHW 2 CHAP 2 - YepezElviraNo ratings yet
- Exercise DispersionDocument10 pagesExercise DispersionMohsin AliNo ratings yet
- Monte Carlo Simulation: Assignment 1Document13 pagesMonte Carlo Simulation: Assignment 1AmeerUlHaqNo ratings yet
- 28 TracerDiffusion PDFDocument11 pages28 TracerDiffusion PDFAkash KumarNo ratings yet
- Metodo de CrossDocument2 pagesMetodo de CrosscristobalNo ratings yet
- Metodo de CrossDocument2 pagesMetodo de CrosscristobalNo ratings yet
- Metodo de CrossDocument2 pagesMetodo de CrossOscar Andreé Fernández RamosNo ratings yet
- ln ΔP percobaan vs ln uDocument4 pagesln ΔP percobaan vs ln uardiNo ratings yet
- Metodo de CrossDocument2 pagesMetodo de CrossSantiago Mohamed Santiago GonzálezNo ratings yet
- Metodo de CrossDocument2 pagesMetodo de CrossJulio GamezNo ratings yet
- Metodo de CrossDocument2 pagesMetodo de CrossRodrigo Peña FernandezNo ratings yet
- Metodo de CrossDocument2 pagesMetodo de Crossluis eca yoveraNo ratings yet
- Metodo de CrossDocument2 pagesMetodo de CrossMariaNo ratings yet
- Edu 2012 Spring MLC Solutions (MLC 09 11)Document152 pagesEdu 2012 Spring MLC Solutions (MLC 09 11)andi.yehudaNo ratings yet
- Iii. Result and Discussion: A. Result Table 3.1 The Result of Measuring of Cardiac Muscle ContractionDocument2 pagesIii. Result and Discussion: A. Result Table 3.1 The Result of Measuring of Cardiac Muscle Contractionpratiwi kusumaNo ratings yet
- Estaca Livre Esforços e DimensionamentoDocument4 pagesEstaca Livre Esforços e DimensionamentoThiago DouradoNo ratings yet
- Lesson 1 KeyDocument21 pagesLesson 1 KeyChristina LeeNo ratings yet
- Chemestry LabDocument6 pagesChemestry LabOsvaldo Izata100% (1)
- DID101Document6 pagesDID101Betty BupNo ratings yet
- Capacidad de Carga (General)Document68 pagesCapacidad de Carga (General)leoNo ratings yet
- Practice Sheet Chemical KineticsDocument1 pagePractice Sheet Chemical KineticsNusrat ZahanNo ratings yet
- Milenka Sela ComDocument30 pagesMilenka Sela ComSergio NinaNo ratings yet
- Milenka Sela ComDocument30 pagesMilenka Sela ComSergio NinaNo ratings yet
- Customer Churn in TelecomDocument9 pagesCustomer Churn in TelecomSubrahmanyam JonnalagaddaNo ratings yet
- Informe 5Document3 pagesInforme 5Abel Mauricio HuamánNo ratings yet
- Tutorial 3Q PDFDocument5 pagesTutorial 3Q PDFLi YuNo ratings yet
- Data Hasil Mohamad FadliDocument7 pagesData Hasil Mohamad FadliMuhammad IkramullahNo ratings yet
- ProkonDocument7 pagesProkonanwarNo ratings yet
- Analytical Modeling of Solute Transport in Groundwater: Using Models to Understand the Effect of Natural Processes on Contaminant Fate and TransportFrom EverandAnalytical Modeling of Solute Transport in Groundwater: Using Models to Understand the Effect of Natural Processes on Contaminant Fate and TransportNo ratings yet
- PDSS 2024Document116 pagesPDSS 2024Ikfal FajarNo ratings yet
- 02 Lampiran I Hasil Psikotes Yang Dinyatakan LulusDocument21 pages02 Lampiran I Hasil Psikotes Yang Dinyatakan LulusIkfal FajarNo ratings yet
- UKBM 3.8 Narative TextDocument15 pagesUKBM 3.8 Narative TextIkfal FajarNo ratings yet
- Pat X Mipa 1Document3 pagesPat X Mipa 1Ikfal FajarNo ratings yet
- Login SiswaDocument21 pagesLogin SiswaIkfal FajarNo ratings yet
- Curriculum Vitae: Examination School/College Board/ University Year of Passing DivisionDocument3 pagesCurriculum Vitae: Examination School/College Board/ University Year of Passing DivisionDhivya KrishnamoorthiNo ratings yet
- DLL Music WEEK 3Document8 pagesDLL Music WEEK 3mariaisabel ignaligNo ratings yet
- Revision MCQ - KinematicsDocument2 pagesRevision MCQ - Kinematicsgaya8404No ratings yet
- Chem 17 - KINETICS OF THE REACTION BETWEEN S2O3 2 - AND H+Document6 pagesChem 17 - KINETICS OF THE REACTION BETWEEN S2O3 2 - AND H+Wilfredo LlanaNo ratings yet
- Weebly English Teacher Resume 2018Document3 pagesWeebly English Teacher Resume 2018api-356295823No ratings yet
- Professional Development Plan 2017-2018Document2 pagesProfessional Development Plan 2017-2018api-353929409No ratings yet
- Lesson Plan in Aquaculture Ncii: Principles in Stocking of Fingerlings and Stock SamplingDocument3 pagesLesson Plan in Aquaculture Ncii: Principles in Stocking of Fingerlings and Stock SamplingAdam WareNo ratings yet
- The Aga Khan Academy Teacher Preparation Program Lesson Observation Template Introductory NotesDocument3 pagesThe Aga Khan Academy Teacher Preparation Program Lesson Observation Template Introductory NotesOliver BayaNo ratings yet
- Chapter 12, Solution 28Document2 pagesChapter 12, Solution 28Mario MartinezNo ratings yet
- Psychological Bases: Bases and Policies of Special and Inclusive EducationDocument18 pagesPsychological Bases: Bases and Policies of Special and Inclusive EducationAlyanna Clarisse Padilla CamposNo ratings yet
- Performance Task For ss10 Term 1Document1 pagePerformance Task For ss10 Term 1api-288515796No ratings yet
- What Are The Benefits of Multigrade Teaching and Learning? Benefits For ChildrenDocument2 pagesWhat Are The Benefits of Multigrade Teaching and Learning? Benefits For ChildrenAleh GirayNo ratings yet
- LAC Session - WHLP and LRCGDocument24 pagesLAC Session - WHLP and LRCGApril Joy DinglasaNo ratings yet
- G11 Free FallDocument25 pagesG11 Free FallDina AlHudrobNo ratings yet
- Lesson Planning Under The New Normal - PowerpointDocument22 pagesLesson Planning Under The New Normal - PowerpointELMAR BANCE67% (3)
- DAILY LESSON LOG (Mathematics)Document3 pagesDAILY LESSON LOG (Mathematics)Karl JamesNo ratings yet
- 2.7.2 - Enzyme Active Site and Substrate Specificity - Biology LibreTextsDocument3 pages2.7.2 - Enzyme Active Site and Substrate Specificity - Biology LibreTextsjamil ahmedNo ratings yet
- Induction Training of Newly Appointed Primary School Teachers-Junior Elementary School Teachers in SindhDocument4 pagesInduction Training of Newly Appointed Primary School Teachers-Junior Elementary School Teachers in SindhwahabNo ratings yet
- Kinetika Kimia s1Document28 pagesKinetika Kimia s1Umiia RuhunussaNo ratings yet
- Assessment For LearningDocument28 pagesAssessment For LearningEne JosephNo ratings yet