Professional Documents
Culture Documents
Excerpt
Uploaded by
Cally ChewOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Excerpt
Uploaded by
Cally ChewCopyright:
Available Formats
Cambridge University Press
978-0-521-18117-4 - Cambridge IGCSE Chemistry Workbook, Third Edition
Richard Harwood and Ian Lodge
Excerpt
More information
1 Planet Earth
Definitions to learn
! acid rain rainfall with a pH usually less than 5 resulting from dissolved atmospheric pollution
! greenhouse gas a gas which absorbs heat (infrared radiation) and keeps the surface of the planet warm
! photosynthesis the photochemical reaction in the green leaves of plants that turns carbon dioxide and
water into glucose and oxygen
! respiration the biochemical reaction in living cells that produces energy from the reaction of glucose
and oxygen to produce carbon dioxide and water
Useful equations
carbon dioxide + water → glucose + oxygen 6CO2 + 6H2O → C6H12O6 + 6O2 photosynthesis
glucose + oxygen → carbon dioxide + water C6H12O6 + 6O2 → 6CO2 + 6H2O respiration
Exercise 1.1 Global warming and the ‘greenhouse effect’
This exercise will help in developing your skills at processing unfamiliar data and making
deductions from novel sources.
The diagram shows a simplified carbon cycle. carbon dioxide in
atmosphere
a Describe the process of photosynthesis
in simple terms.
combustion and
photosynthesis
respiration
.............................................................................. fossil
fuels
oceans
..............................................................................
limestone sediments
..............................................................................
The ‘greenhouse effect’ is caused by heat from the Sun being trapped inside the Earth’s
atmosphere by some of the gases which are present – their molecules absorb infrared
radiation. As the amount of these ‘greenhouse gases’ increases, the mean (average)
temperature of the Earth increases. It is estimated that if there were no ‘greenhouse effect’
the Earth’s temperature would be cooler by 33 °C on average. Some of the gases which cause
this effect are carbon dioxide, methane and oxides of nitrogen (NOx).
1 Planet Earth 1
© in this web service Cambridge University Press www.cambridge.org
Cambridge University Press
978-0-521-18117-4 - Cambridge IGCSE Chemistry Workbook, Third Edition
Richard Harwood and Ian Lodge
Excerpt
More information
Global warming: Since the burning of fossil fuels started to increase in the late 19th century,
the amount of carbon dioxide in the atmosphere has increased steadily. The changes in
the mean temperature of the Earth have not been quite so regular. Below are some data
regarding the changes in mean temperature of the Earth and amount of carbon dioxide in
the atmosphere. The first table (Table 1) gives the changes over recent years, while the second
table gives the longer term changes (Table 2). The mean temperature is the average over all
parts of the Earth’s surface over a whole year. The amount of carbon dioxide is given in ppm
(parts of carbon dioxide per million parts of air).
Mean Mean
temperature temperature
Year CO2 / ppm / ºC Year CO2 / ppm / ºC
1980 338 14.28 1880 291 13.92
1982 340 14.08 1890 294 13.81
1984 343 14.15 1900 297 13.95
1986 347 14.19 1910 300 13.80
1988 351 14.41 1920 303 13.82
1990 354 14.48 1930 306 13.96
1992 356 14.15 1940 309 14.14
1994 358 14.31
1950 312 13.83
1996 361 14.36
1998 366 14.70 1960 317 13.99
2000 369 14.39 1970 324 14.04
2002 373 14.67 1980 338 14.28
2004 377 14.58 Table 2
2006 381 14.63
2008 385 14.51
Table 1
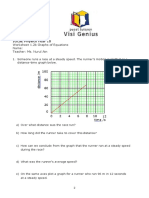
b Plot these results on the grid using the left-hand y-axis for amount of carbon dioxide and
the right-hand y-axis for mean temperature. Draw two separate graphs to enable you to
compare the trends (use graph paper if you need a larger grid).
390 15.0
380 14.8
370 14.6
360 14.4
Mean temperature / °C
Carbon dioxide / ppm
350 14.2
340 14.0
330 13.8
320 13.6
310 13.4
300 13.2
290 13.0
1880 1900 1920 1940 1960 1980 2000 2020 2040
Year
2 IGCSE Chemistry
© in this web service Cambridge University Press www.cambridge.org
Cambridge University Press
978-0-521-18117-4 - Cambridge IGCSE Chemistry Workbook, Third Edition
Richard Harwood and Ian Lodge
Excerpt
More information
Use the check list below to give yourself a mark for your graph.
For each point, award yourself: 2 marks if you did it really well
1 mark if you made a good attempt at it, and partly succeeded
0 marks if you did not try to do it, or did not succeed.
Self-assessment check list for a graph:
Marks awarded
Your
Check point You
teacher
You have plotted each point precisely and correctly for both sets
of data; using the different scales on the two vertical axes.
You have used a small, neat cross or dot for the points of one graph.
You have used a small, but different, symbol for the points of the
other graph.
You have drawn the connecting lines through one set of points
accurately – using a ruler for the lines.
You have drawn the connecting lines through the other set of points
accurately – using a different colour or broken line.
You have ignored any anomalous results when drawing the lines.
Total (out of 12)
10–12 Excellent.
7–9 Good.
4–6 A good start, but you need to improve quite a bit.
2–3 Poor. Try this same graph again, using a new sheet of graph paper.
1 Very poor. Read through all the criteria again, and then try the same
graph again.
c What do you notice about the trend in amount of carbon dioxide?
.........................................................................................................................................................
.........................................................................................................................................................
d What do you notice about the trend in mean temperature?
.........................................................................................................................................................
.........................................................................................................................................................
1 Planet Earth 3
© in this web service Cambridge University Press www.cambridge.org
Cambridge University Press
978-0-521-18117-4 - Cambridge IGCSE Chemistry Workbook, Third Edition
Richard Harwood and Ian Lodge
Excerpt
More information
e Does the graph clearly show that an increase in carbon dioxide is causing an increase in
temperature?
.........................................................................................................................................................
.........................................................................................................................................................
f Estimate the amount of carbon dioxide in the atmosphere and the likely mean
temperature of the Earth in the years 2020 and 2040.
.........................................................................................................................................................
.........................................................................................................................................................
g Between the 11th century and the end of the 18th century the amount of carbon dioxide
in the atmosphere varied between 275 and 280 ppm. Why did it start to rise from the
19th century onwards.
.........................................................................................................................................................
h Other ‘greenhouse gases’ are present in much smaller amounts. However they are much
more effective at keeping in heat than carbon dioxide. Methane (1.7 ppm) has 21 times
the effect of carbon dioxide. Nitrogen oxides (0.3 ppm) have 310 times the effect of
carbon dioxide.
Name a source that releases each of these gases into the atmosphere.
methane: .........................................................................................................................................
nitrogen oxides: .............................................................................................................................
Exercise 1.2 Hydrogen as a fuel
This exercise introduces an alternative energy source and will help develop your skills at
handling information regarding unfamiliar applications.
One of the first buses to use hydrogen as a fuel was operated in Erlangen, Germany, in
1996. The hydrogen was stored in thick pressurised tanks on the roof of the bus.
a Describe two advantages of using hydrogen as a fuel rather than gasoline (petrol).
.........................................................................................................................................................
.........................................................................................................................................................
b Suggest one disadvantage of using hydrogen as a fuel.
.........................................................................................................................................................
4 IGCSE Chemistry
© in this web service Cambridge University Press www.cambridge.org
Cambridge University Press
978-0-521-18117-4 - Cambridge IGCSE Chemistry Workbook, Third Edition
Richard Harwood and Ian Lodge
Excerpt
More information
S It is possible to generate electrical energy from hydrogen using a fuel cell. The structure
of a typical fuel cell is shown in the diagram.
external
circuit
hydrogen oxygen
electrolyte
gas gas
porous carbon porous carbon
anode cathode
water
c The reaction taking place in such a fuel cell is the combustion of hydrogen. Write the
overall equation for that reaction.
.........................................................................................................................................................
d The equation for the reaction at the anode is
H2(g) + 2OH−(aq) → 2H2O(l) + 2e−
What type of reaction is this? Explain your answer.
.........................................................................................................................................................
e At the cathode oxygen molecules react with water molecules to form hydroxide ions.
Write an ionic equation for this reaction.
.........................................................................................................................................................
1 Planet Earth 5
© in this web service Cambridge University Press www.cambridge.org
Cambridge University Press
978-0-521-18117-4 - Cambridge IGCSE Chemistry Workbook, Third Edition
Richard Harwood and Ian Lodge
Excerpt
More information
2 The nature of matter
Definitions to learn
! physical state the three states of matter are solid, liquid and gas
! condensation the change of state from gas to liquid
! melting the change of state from solid to liquid
! freezing the change of state from liquid to solid at the melting point
! boiling the change of state from liquid to gas at the boiling point of the liquid
! evaporation the change of state from liquid to gas below the boiling point
! sublimation the change of state directly from solid to gas (or the reverse)
! crystallisation the formation of crystals when a saturated solution is left to cool
! filtration the separation of a solid from a liquid using filter paper
! distillation the separation of a liquid from a mixture using differences in boiling point
! fractional distillation the separation of a mixture of liquids using differences in boiling point
! diffusion the random movement of particles in a fluid (liquid or gas) leading to the complete mixing
of the particles
! chromatography the separation of a mixture of soluble (coloured) substances using paper and a solvent
! atom the smallest part of an element that can take part in a chemical change
! proton number (atomic number) the number of protons in the nucleus of an atom of an element
! nucleon number (mass number) the number of protons and neutrons in the nucleus of an atom
! electron arrangement the organisation of electrons in their different energy levels (shells)
! isotopes atoms of the same element with different numbers of neutrons in their nuclei
Exercise 2.1 Changing physical state
This exercise will develop your understanding of the kinetic theory and the energy changes
involved in changes of physical state.
The graph shows the heating curve for
a pure substance. The temperature rises
D
with time as the substance is heated.
Temperature / ºC
115
C
B
17
A
0
0 Time
6 IGCSE Chemistry
© in this web service Cambridge University Press www.cambridge.org
Cambridge University Press
978-0-521-18117-4 - Cambridge IGCSE Chemistry Workbook, Third Edition
Richard Harwood and Ian Lodge
Excerpt
More information
a What physical state(s) is the substance in at points A, B, C and D?
A .......................................... C ..........................................
B .......................................... D ..........................................
b What is the melting point of the substance? ..........................................
c What is its boiling point? ..........................................
d What happens to the temperature while the substance is changing state?
.........................................................................................................................................................
e The substance is not water. How do we know this from the graph?
.........................................................................................................................................................
f Complete the passage using the words given below.
different diffusion gas spread particles
diffuse random lattice vibrate temperature
The kinetic theory states that the .......................................... in a liquid and a
.......................................... are in constant motion. In a gas the particles are far apart from
each other and their motion is said to be .......................................... . The particles in a
solid are held in fixed positions in a regular .......................................... . In a solid the
particles can only .......................................... about their fixed positions.
Liquids and gases are fluid states. When particles move in a fluid they can collide with
each other. When they collide they bounce off each other in ..........................................
directions. If two gases or liquids are mixed then the different types of particle
.......................................... out and get mixed up. This process is called .......................................... .
S At the same .......................................... particles that have a lower mass move faster than those
with higher mass. This means that the lighter particles will spread and mix more quickly; the
lighter particles are said to .......................................... faster than the heavier particles.
2 The nature of matter 7
© in this web service Cambridge University Press www.cambridge.org
Cambridge University Press
978-0-521-18117-4 - Cambridge IGCSE Chemistry Workbook, Third Edition
Richard Harwood and Ian Lodge
Excerpt
More information
g Use the data given for the substances listed below to decide which of them will be solids,
liquids or gases at a room temperature of 25 °C and atmospheric pressure.
Substance Melting point / °C Boiling point / °C
sodium 98 883
radon −71 −62
ethanol −117 78
cobalt 1492 2900
nitrogen −210 −196
propane −188 −42
ethanoic acid 16 118
i Which substance is a liquid over the smallest range of temperature? ......................................
ii Which two substances are gaseous at −50 °C? .......................................... and
..........................................
iii Which substance has the lowest freezing point? ..........................................
iv Which substance is liquid at 2500 °C? ..........................................
v A sample of ethanoic acid was found to boil at 121 °C at atmospheric pressure. Use the
information in the table to comment on this result.
..........................................................................................................................................................
..........................................................................................................................................................
Exercise 2.2 Chromatography at the races
This exercise will help you understand aspects of chromatography by considering an
unfamiliar application of the technique.
Chromatography is used by the ‘Horse Racing Forensic Laboratory’ to test for the presence of
illegal drugs in racehorses.
A concentrated sample of urine is spotted onto chromatography paper on the start line.
Alongside this, known drugs are spotted. The chromatogram is run using methanol as the
solvent. When finished, the paper is read by placing it under ultraviolet light. A chromatogram of
urine from four racehorses is shown on the next page.
8 IGCSE Chemistry
© in this web service Cambridge University Press www.cambridge.org
Cambridge University Press
978-0-521-18117-4 - Cambridge IGCSE Chemistry Workbook, Third Edition
Richard Harwood and Ian Lodge
Excerpt
More information
10 solvent front Spot Description
9
8
1 caffeine
7 2 paracetamol
6
Scale / cm
5 3 urine sample horse A
4
4 urine sample horse B
3
2 5 urine sample horse C
1
0 start line
6 urine sample horse D
1 2 3 4 5 6
a State two factors which determine the distance a substance travels up the paper.
.........................................................................................................................................................
.........................................................................................................................................................
b From the results, the sample from one horse contains an illegal substance. State which
horse, and the drug present.
.........................................................................................................................................................
c Give a reason for the use of this drug.
.........................................................................................................................................................
S d The results for known drugs are given as ‘Rf values’.
distance travelled by the substance
Rf =
distance travelleed by the solvent
Calculate the Rf value for caffeine.
2 The nature of matter 9
© in this web service Cambridge University Press www.cambridge.org
Cambridge University Press
978-0-521-18117-4 - Cambridge IGCSE Chemistry Workbook, Third Edition
Richard Harwood and Ian Lodge
Excerpt
More information
Exercise 2.3 Atomic structure
This exercise helps familiarise you with aspects of atomic structure and the uses
of radioactivity.
a Choose from the words below to fill in the gaps in the passage. Words may be used once,
more than once or not at all.
proton electrons nucleon isotopes protons
neutrons nucleus energy levels
Atoms are made up of three different particles: ..........................................
which are positively charged; .......................................... which have no charge;
and .......................................... which are negatively charged. The negatively charged
particles are arranged in different .......................................... (shells) around the
.......................................... of the atom. The particles with a negligible mass are
the ......................................... . All atoms of the same element contain the same number of
.......................................... and ........................................... . Atoms of the same element with
different numbers of .......................................... are known as ........................................... .
b This part of the exercise is concerned with electron arrangements and the structure of the
Periodic Table. Complete these sentences by filling in the blanks with words or numbers.
The electrons in an atom are arranged in a series of ........................................... around the
nucleus. These shells are also called ........................................... levels. In an atom the shell
........................................... to the nucleus fills first, then the next shell, and so on. There is
room for
● up to ........... electrons in the first shell
● up to ........... electrons in the second shell
● up to ........... electrons in the third shell.
(There are 18 electrons in total when the three shells are completely full).
10 IGCSE Chemistry
© in this web service Cambridge University Press www.cambridge.org
Cambridge University Press
978-0-521-18117-4 - Cambridge IGCSE Chemistry Workbook, Third Edition
Richard Harwood and Ian Lodge
Excerpt
More information
The elements in the Periodic Table are organised in the same way as the electrons fill the
shells. Shells fill from ........................................... to ........................................... across
the ........................................... of the Periodic Table.
● The first shell fills up first from ........................................... to helium.
● The second shell fills next from lithium to ............................................ .
● Eight ........................................... go into the third shell from sodium to argon.
● Then the fourth shell starts to fill from potassium.
c In 1986, an explosion at Chernobyl in the Ukraine released a radioactive cloud
containing various radioactive isotopes. Three such isotopes are shown in the table. Use
your Periodic Table to answer the following questions.
Element Nucleon (mass) number
strontium 90
iodine 131
caesium 137
i How many electrons are there in one atom of strontium-90? ..........................................
ii How many protons are there in one atom of iodine-131? ...........................................
iii How many neutrons are there in an atom of caesium-137? ...........................................
The prevailing winds carried fall-out from Chernobyl towards Scandinavia. In Sweden,
caesium-137 built up in lichen which is the food eaten by reindeer. This gave rise to
radioactive meat.
iv If radioactive caesium was reacted with chlorine, would you expect the caesium
chloride produced to be radioactive? Explain your answer.
...................................................................................................................................................
v State a beneficial use in industry of a radioactive isotope.
...................................................................................................................................................
vi State a medical use of a radioactive isotope.
...................................................................................................................................................
2 The nature of matter 11
© in this web service Cambridge University Press www.cambridge.org
You might also like
- Presentation On OZONEDocument138 pagesPresentation On OZONEYou TubeNo ratings yet
- Climatic PhenomenaDocument21 pagesClimatic PhenomenaRHEAMAE GALLEGONo ratings yet
- Global Worming and Greenhose Gases PDFDocument5 pagesGlobal Worming and Greenhose Gases PDFShubham Raj SanuNo ratings yet
- Amalie Noergaard - Climate Change Test ReviewDocument6 pagesAmalie Noergaard - Climate Change Test ReviewRegina RosadoNo ratings yet
- Global WarmingDocument14 pagesGlobal WarmingShehzad Afzal MaharNo ratings yet
- Greenhouse Gases 2017Document11 pagesGreenhouse Gases 2017AnusheenNo ratings yet
- Greenhouse Effect: Learning OutcomeDocument16 pagesGreenhouse Effect: Learning OutcomeJames WongNo ratings yet
- Cider CarbonFootprintDocument13 pagesCider CarbonFootprintdmurenNo ratings yet
- Green Houses and Climate ChangeDocument25 pagesGreen Houses and Climate ChangeFre DocsNo ratings yet
- Global WarmingDocument10 pagesGlobal WarmingYashraj RathiNo ratings yet
- Assignment of CombustionDocument9 pagesAssignment of CombustionAfiq de WinnerNo ratings yet
- Climate ChangeDocument25 pagesClimate ChangeRahul MaliNo ratings yet
- Climate Change, and The Scientific ProcessDocument25 pagesClimate Change, and The Scientific ProcessUmar FarooqNo ratings yet
- Geography Christmas Assignment 2020-Laptop-Irhdj86iDocument11 pagesGeography Christmas Assignment 2020-Laptop-Irhdj86iJaiden LebrunNo ratings yet
- Climate Change 1Document102 pagesClimate Change 1Akiko Princess Galagar100% (1)
- Cambridge IGCSE Chemistry Topic 11: Air and WaterDocument3 pagesCambridge IGCSE Chemistry Topic 11: Air and WaterPatuan TampuolonNo ratings yet
- ADITI VERMA (Assignment 1, SUSTAINABLE ARCHITECTURE)Document30 pagesADITI VERMA (Assignment 1, SUSTAINABLE ARCHITECTURE)Sani SahaniNo ratings yet
- The EarthDocument3 pagesThe EarthloheesNo ratings yet
- Geological Sequestration of Carbon Dioxide: Thermodynamics, Kinetics, and Reaction Path ModelingFrom EverandGeological Sequestration of Carbon Dioxide: Thermodynamics, Kinetics, and Reaction Path ModelingNo ratings yet
- Greenhouse Effect and Global Warming: Assignment#01Document11 pagesGreenhouse Effect and Global Warming: Assignment#01Abeerataimur AbeerataimurNo ratings yet
- Green House EffectDocument6 pagesGreen House EffectVir PatelNo ratings yet
- Greenhouse EffectDocument30 pagesGreenhouse EffectFernan SibugNo ratings yet
- 13-3 Global Warming PDFDocument15 pages13-3 Global Warming PDFRocío RomeroNo ratings yet
- Calentamiento GlobalDocument5 pagesCalentamiento GlobalMitzi EstradaNo ratings yet
- 1111 Bird-112Document32 pages1111 Bird-112Dipanjan DasNo ratings yet
- 1111 Brandingsuccessstories-12Document30 pages1111 Brandingsuccessstories-12Dipanjan DasNo ratings yet
- Greenhouse EffectDocument12 pagesGreenhouse Effectjagdish002No ratings yet
- Greenhouse EffectDocument8 pagesGreenhouse EffectAmple CunananNo ratings yet
- Kimberly Montanez Assignment1 1 (A, B) 1 4 EngDocument2 pagesKimberly Montanez Assignment1 1 (A, B) 1 4 EngkimberlyNo ratings yet
- Be Skeptical Be Very Skeptical !Document18 pagesBe Skeptical Be Very Skeptical !Dipanjan DasNo ratings yet
- Expose Anlais Groupe 9Document7 pagesExpose Anlais Groupe 9Delano TamkoNo ratings yet
- BIOL 002 Global Warming: Beirut Arab University Faculty of Science Department of BiologyDocument65 pagesBIOL 002 Global Warming: Beirut Arab University Faculty of Science Department of BiologyYoumna ShatilaNo ratings yet
- PP 31 The Atmosphere and Climate ChangeDocument97 pagesPP 31 The Atmosphere and Climate ChangeOlerato NtsimaneNo ratings yet
- Topic 2: Elemental and Environmental ChemistryDocument43 pagesTopic 2: Elemental and Environmental ChemistryChangWeiTanNo ratings yet
- Module 7 - Kiss NotesDocument23 pagesModule 7 - Kiss Noteszachbartlett3No ratings yet
- Explanation TeksDocument4 pagesExplanation TeksDaniel SitanggangNo ratings yet
- University Sinergija Faculty of Security and Protection Banja LukaDocument16 pagesUniversity Sinergija Faculty of Security and Protection Banja LukaSasa DjurdjevicNo ratings yet
- Preview Book2Document9 pagesPreview Book2elenaNo ratings yet
- Climate Science IYCNDocument41 pagesClimate Science IYCNChaitanya KumarNo ratings yet
- Science9 Q3 SLM13Document11 pagesScience9 Q3 SLM13alberto.deuna001No ratings yet
- L5 CCST9023 ClimateDocument84 pagesL5 CCST9023 ClimateDaniel RadcliffNo ratings yet
- English Compulsory MaterialDocument8 pagesEnglish Compulsory MaterialMaryam ZaidiNo ratings yet
- Carbon Dioxide Is A Greenhouse Gas That Makes A Planet WarmerDocument4 pagesCarbon Dioxide Is A Greenhouse Gas That Makes A Planet WarmerNavneet SinghNo ratings yet
- What Are The Reasons For Global Warming?Document6 pagesWhat Are The Reasons For Global Warming?manirathinaNo ratings yet
- The Greenhouse Effect and Global WarmingDocument20 pagesThe Greenhouse Effect and Global WarmingNimra KhalidNo ratings yet
- Chem 2 Chemistry in Your World 2Nd Edition Hogg Test Bank Full Chapter PDFDocument28 pagesChem 2 Chemistry in Your World 2Nd Edition Hogg Test Bank Full Chapter PDFcarolyn.leung589100% (14)
- Global WarmingDocument7 pagesGlobal WarmingHardik TankNo ratings yet
- Boxes and PackagingDocument1 pageBoxes and PackaginggabrielNo ratings yet
- Climate Emergency PamphletDocument52 pagesClimate Emergency PamphletClimate CampaignerNo ratings yet
- Climate Change PPT 1Document17 pagesClimate Change PPT 1api-573214664No ratings yet
- Global Warming DebateDocument2 pagesGlobal Warming DebateAry WulanNo ratings yet
- Global Warming: Problem QuestionDocument8 pagesGlobal Warming: Problem QuestionClairNo ratings yet
- Green House Effect .Document37 pagesGreen House Effect .ZXaiba Zxafr100% (2)
- L1 4 Exam Qu NTDocument12 pagesL1 4 Exam Qu NTmelissaicecream10No ratings yet
- Globle WarmingDocument87 pagesGloble WarmingVijay Maurya100% (1)
- Chapter 6 Global Environment IssuesDocument31 pagesChapter 6 Global Environment IssuesCupcakeNo ratings yet
- Earth System 3rd Edition Kump Solutions ManualDocument8 pagesEarth System 3rd Edition Kump Solutions Manualjessicaramseymxpqrcbgjt100% (8)
- Mass and WeightDocument3 pagesMass and WeightCally ChewNo ratings yet
- Balanced and Unbalanced ForceDocument29 pagesBalanced and Unbalanced ForceJulie Anne Delos ReyesNo ratings yet
- PDF Document 2176D71B14AE 1Document10 pagesPDF Document 2176D71B14AE 1Cally ChewNo ratings yet
- Elements, Compounds,&MixturesDocument20 pagesElements, Compounds,&MixturesCally ChewNo ratings yet
- PDF document-56D450CF704E-1Document19 pagesPDF document-56D450CF704E-1Cally ChewNo ratings yet
- ExcerptDocument10 pagesExcerptCally ChewNo ratings yet
- Specialized Cells Extra ChallengeDocument1 pageSpecialized Cells Extra ChallengeCally ChewNo ratings yet
- PDF document-49F9440362E1-1Document12 pagesPDF document-49F9440362E1-1Cally ChewNo ratings yet
- Human HealthDocument31 pagesHuman Health2375606245No ratings yet
- Pop Quiz - Forces and MotionDocument2 pagesPop Quiz - Forces and MotionNurulAinMatAron50% (2)
- Worksheet 1.2bDocument5 pagesWorksheet 1.2bNurulAinMatAron100% (1)
- 巴生中华 毕业考1 国文Document15 pages巴生中华 毕业考1 国文Cally ChewNo ratings yet
- PDF document-B36436539DE9-1Document23 pagesPDF document-B36436539DE9-1Cally ChewNo ratings yet
- Year 7 Variation-WorksheetDocument17 pagesYear 7 Variation-WorksheetCally ChewNo ratings yet
- Animal and Plant Cell QuestionsDocument5 pagesAnimal and Plant Cell QuestionsFaiar Rob Year 9No ratings yet
- 4.4.2.1 GCSE Biology. AQA OCR EDEXCEL. Aerobic Respiration AnswersDocument4 pages4.4.2.1 GCSE Biology. AQA OCR EDEXCEL. Aerobic Respiration AnswersCally ChewNo ratings yet
- 4.7.2.2 GCSE Biology. AQA OCR EDEXCEL. Material Cycling AnswersDocument4 pages4.7.2.2 GCSE Biology. AQA OCR EDEXCEL. Material Cycling AnswersCally ChewNo ratings yet
- 4.2.2.2 GCSE Biology AQA OCR EDEXCEL. Heart and Blood Vessels AnswersDocument4 pages4.2.2.2 GCSE Biology AQA OCR EDEXCEL. Heart and Blood Vessels AnswersCally ChewNo ratings yet
- 4.2.2.3 Gcse Biology Aqa Ocr Edexcel. Blood AnswersDocument5 pages4.2.2.3 Gcse Biology Aqa Ocr Edexcel. Blood AnswersCally ChewNo ratings yet
- 4.2.2.4 GCSE Biology AQA OCR EDEXCEL. Coronary Heart Disease AnswersDocument4 pages4.2.2.4 GCSE Biology AQA OCR EDEXCEL. Coronary Heart Disease AnswersCally ChewNo ratings yet
- 4.2.1 GCSE Biology AQA OCR EDEXCEL. Principles of Organisation AnswersDocument4 pages4.2.1 GCSE Biology AQA OCR EDEXCEL. Principles of Organisation AnswersCally ChewNo ratings yet
- Grade 8 Integrated Science Week 9 Lesson 1 Worksheets 1 and AnswersheetsDocument4 pagesGrade 8 Integrated Science Week 9 Lesson 1 Worksheets 1 and AnswersheetsCally ChewNo ratings yet
- 4.7.2.2 GCSE Biology. AQA OCR EDEXCEL. Material Cycling AnswersDocument4 pages4.7.2.2 GCSE Biology. AQA OCR EDEXCEL. Material Cycling AnswersCally ChewNo ratings yet
- 4.4.2.1 GCSE Biology. AQA OCR EDEXCEL. Aerobic Respiration AnswersDocument4 pages4.4.2.1 GCSE Biology. AQA OCR EDEXCEL. Aerobic Respiration AnswersCally ChewNo ratings yet
- 4.2.2.3 Gcse Biology Aqa Ocr Edexcel. Blood AnswersDocument5 pages4.2.2.3 Gcse Biology Aqa Ocr Edexcel. Blood AnswersCally ChewNo ratings yet
- Acid, Alkali Ext Year 8Document3 pagesAcid, Alkali Ext Year 8Cally ChewNo ratings yet
- Grade 7 Integrated Science Week 6 Lesson 1Document3 pagesGrade 7 Integrated Science Week 6 Lesson 1Cally ChewNo ratings yet
- Electricity (Note)Document9 pagesElectricity (Note)Cally ChewNo ratings yet
- Year-7-Science-revision 2Document17 pagesYear-7-Science-revision 2Cally ChewNo ratings yet
- Science 9 Unit D Section 1 Electricity PPT Cherkas 2013Document44 pagesScience 9 Unit D Section 1 Electricity PPT Cherkas 2013Cally ChewNo ratings yet
- Effect of Petrol Fumes On An Anthropometry and Ventilatory Function Among Petrol Pump Workers of Puducherry, IndiaDocument13 pagesEffect of Petrol Fumes On An Anthropometry and Ventilatory Function Among Petrol Pump Workers of Puducherry, IndiaABHINABA GUPTANo ratings yet
- Budget of Work Sci5Document1 pageBudget of Work Sci5Jhoy Angeles PinlacNo ratings yet
- Solutionbank D1: Edexcel AS and A Level Modular MathematicsDocument30 pagesSolutionbank D1: Edexcel AS and A Level Modular MathematicsMaruf_007No ratings yet
- Data Visualization For Python - Sales Retail - r1Document19 pagesData Visualization For Python - Sales Retail - r1Mazhar MahadzirNo ratings yet
- Watchgas AirWatch MK1.0 Vs MK1.2Document9 pagesWatchgas AirWatch MK1.0 Vs MK1.2elliotmoralesNo ratings yet
- Technical Data: Series Allclean AcnpDocument1 pageTechnical Data: Series Allclean AcnpBoško IvanovićNo ratings yet
- College of Technology & Engineering: Practical Training at Hindustan Zinc Limited Zinc Smelter, Debari UdaipurDocument24 pagesCollege of Technology & Engineering: Practical Training at Hindustan Zinc Limited Zinc Smelter, Debari UdaipurPooja SahuNo ratings yet
- North Sails Brochure 2007 enDocument24 pagesNorth Sails Brochure 2007 ennorthsailsNo ratings yet
- Chapter 16 - Oral Radiography (Essentials of Dental Assisting)Document96 pagesChapter 16 - Oral Radiography (Essentials of Dental Assisting)mussanteNo ratings yet
- Harmony XB4 - XB4BD53Document5 pagesHarmony XB4 - XB4BD53vNo ratings yet
- The Joyce of Science - ConclusionDocument2 pagesThe Joyce of Science - ConclusionAhmadNo ratings yet
- Allison 1,000 & 2,000 Group 21Document4 pagesAllison 1,000 & 2,000 Group 21Robert WhooleyNo ratings yet
- P 130881757895329843Document44 pagesP 130881757895329843Vijay MohanNo ratings yet
- Varargout Tugas - GUI (Varargin) : FunctionDocument7 pagesVarargout Tugas - GUI (Varargin) : FunctionDwi Lestari dwi375ft.2019No ratings yet
- Plagiarism - ReportDocument6 pagesPlagiarism - ReportDipesh NagpalNo ratings yet
- Grade 12 Differentiation CHPT 7 & 8Document60 pagesGrade 12 Differentiation CHPT 7 & 8Sri Devi NagarjunaNo ratings yet
- Ex450-5 Technical DrawingDocument12 pagesEx450-5 Technical DrawingTuan Pham AnhNo ratings yet
- Unit Exam 5Document3 pagesUnit Exam 5Rose AstoNo ratings yet
- 1.summative-Test Math7Document1 page1.summative-Test Math7Jaylor GaridoNo ratings yet
- List of GHS Hazard Statement & PictogramsDocument33 pagesList of GHS Hazard Statement & PictogramsKhairul BarsriNo ratings yet
- Simple Harmonic Oscillator: 1 HamiltonianDocument10 pagesSimple Harmonic Oscillator: 1 HamiltonianAbdurrahman imamNo ratings yet
- Che 410 ................... Transition Metal ChemistryDocument13 pagesChe 410 ................... Transition Metal ChemistryElizabeth AnyangoNo ratings yet
- Ny-Damecaax500 Brochure Juli-2019Document8 pagesNy-Damecaax500 Brochure Juli-2019Shavin FernandoNo ratings yet
- Various Image Enhancement Techniques-A Critical Review: S.S. Bedi, Rati KhandelwalDocument5 pagesVarious Image Enhancement Techniques-A Critical Review: S.S. Bedi, Rati KhandelwalArina AndriesNo ratings yet
- Chapter VI DP and NetworkDocument66 pagesChapter VI DP and NetworkSirgut TesfayeNo ratings yet
- C191HM Powermeter and Harmonic Manager CommunicationsDocument30 pagesC191HM Powermeter and Harmonic Manager CommunicationsRoberto GarridoNo ratings yet
- Narayana Xii Pass Ir Iit (2023 24) PDFDocument16 pagesNarayana Xii Pass Ir Iit (2023 24) PDFRaghav ChaudharyNo ratings yet
- Pressure Sensor Air PST Datasheet 51 en 2780071435Document3 pagesPressure Sensor Air PST Datasheet 51 en 2780071435Luis GuevaraNo ratings yet
- Ecoflam Burners 2014 enDocument60 pagesEcoflam Burners 2014 enanonimppNo ratings yet
- TMT Boron CoatingDocument6 pagesTMT Boron Coatingcvolkan1100% (2)