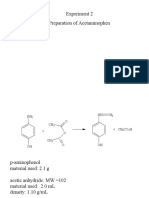
Professional Documents
Culture Documents
DC10962
Uploaded by
and12345Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
DC10962
Uploaded by
and12345Copyright:
Available Formats
Steam Distillation of Cinnamon
Introduction
Essential oils have been used since ancient times as perfumes, flavorings and even medicines.
So-called because they seem to contain the odor and flavor “essence” of a plant, essential oils SCIENTIFIC
are volatile organic liquids obtained from flowers, leaves, twigs, fruits, nuts or seeds.
Concepts
• Essential oil • Steam distillation
• Solvent extraction • Aldehyde functional group
Background
There are three main methods for obtaining essential oils from plants: steam distillation, extraction with a solvent, and
expression or cold pressing. Steam distillation involves heating plant components with water or steam and collecting the liq-
uid distillate. The latter consists of an immiscible mixture of water and the essential oil.
When a mixture of two immiscible liquids, such as water and oil, is heated, each liquid exerts its vapor pressure independently of
the other liquid. The total vapor pressure (Ptotal) at a given temperature is therefore equal to the sum of the vapor pressures of
the two immiscible liquids. Equation 1 describes the total vapor pressure for a mixture of an essential oil and water.
Ptotal = P H + P Oil Equation 1
2O
A mixture of two immiscible liquids will boil when their combined vapor pressure is equal to 760 mm Hg. The boiling
point of a mixture of water and essential oil will thus be less than the boiling point of either component separately. This is
an advantage in the distillation of natural products that have high boiling points and would decompose at high
temperatures.
The amounts of water and essential oil that will co-distill during steam distillation can be determined using Equation 1
and the ideal gas law. The mole ratio of oil and water in the distillate is equal to their relative vapor pressures (P) at the
boiling point (Equation 2, n = number of moles).
n(oil) P oil
——— = ——— Equation 2
n(H2O) PH O
2
Multiplying each term in Equation 2 by the molar mass (MM, g/mole) of the substance gives the mass of water in
grams that will co-distill with an essential oil (Equation 3).
n(oil) × MM(oil) mass of oil (g) P × MM (oil)
————————— = ——————— = ————————– oil Equation 3
n(H2O) × MM (H2O) mass of water (g) P H O × MM (water)
2
Cinnamon is obtained from the inner bark of Cinnamomum zeylanicum, a small evergreen that is native to Sri Lanka and
India. Oil of cinnamon is obtained from cinnamon bark by steam distillation. The major componant of oil of cinnamon is
cinnamaldehyde (70–80%). Cinnamaldehyde is classified as an aromatic aldehyde—it contains an aldehyde functional group
and has a benzene (aromatic) ring (see Figure 1). The compound has natural antimicrobial properties but is highly
irritating to the skin. Minor components of oil of cinnamon include eugenol (10–20%) and cinnamic acid (5–10%).
© 2016 Flinn Scientific, Inc. All Rights Reserved.
Publication No. 10962 1
061616
Steam Distillation of Cinnamon
O Aldehyde functional group
H H H
H C H CO2H
H C C C H H C CH2CH CH2 H C C C
C C H C C C C H
C C C C C C
H C H HO C H H C H
Aromatic ring H OCH3 H
Cinnamaldehyde Eugenol Cinnamic Acid
Figure 1. Components of Oil of Cinnamon.
The purpose of this activity is to isolate oil of cinnamon by steam distillation and study its chemical properties. The mixture of
oil and water in the distillate will be extracted with hexane to dissolve the oil and the solvent will then be evaporated. The
pres- ence of cinnamaldehyde can be identified using a qualitative test.
Materials (for each student group)
Cinnamaldehyde, 0.5 mL Erlenmeyer flask, 125-mL
Cinnamon sticks, fresh, 10 g Heating mantle and variable transformer
Hexane, 15 mL Round-bottom flask, 250-mL
Schiff reagent, 2 mL Round-bottom flask, 100-mL
Sodium chloride, 10 g Rubber tubing, 2 pieces
Water Separatory funnel, 125-mL
Custom distillation head (condenser and adapters) Stirring rod
Hot plate Stopcock grease (optional)
Thermometer and thermometer adapter Support (ring) stands, 2
Beral pipets, 3 Test tube, large
Beaker, 400-mL Test tubes, small, 2
Clamps, 2 Weighing dish, large
Erlenmeyer flasks, 50-mL, 2
Safety Precautions
Oil of cinnamon and cinnamaldehyde are severe skin irritants. Hexane is a flammable liquid and a dangerous fire risk—do not use around
flames. Avoid contact of all chemicals with eyes and skin. Wear chemical splash goggles, chemical-resistant gloves, and a chemical-resistant
apron. Please review current Safety Data Sheets for additional safety, handling, and disposal information. Wash hands thoroughly with soap
and water before leaving the laboratory.
Procedure
1. Obtain about 10 g of fresh cinnamon sticks in a large weighing dish and break the sticks into small pieces.
2. Add the broken cinnamon pieces to a 250-mL round-bottom flask and fill the flask about one-half full with water. This is
the distilling flask.
3. Place the distilling flask into a heating mantle and clamp the flask to a support (ring) stand.
4. Place the one-piece custom distillation head (or a three-way adapter, followed by a condenser and a second adapter)
into the distilling flask (see Figure 2).
5. Place the thermometer holder into the adapter in the distillation head. Carefully insert the thermometer so that the
ther- mometer bulb rests just below the side arm that leads to the condenser.
6. Place a 100-mL round-bottom flask into the side-arm adapter attached to the distillation head. Gently clamp the
receiving flask to a support stand. Cool the flask in an ice bath.
2 © 2016 Flinn Scientific, Inc. All Rights
Steam Distillation of Cinnamon
7. Attach one piece of rubber tubing from the inlet nozzle on the condenser to the faucet, and place the second piece of
rubber tubing from the outlet nozzle on the condenser into the sink to drain.
8. Turn on the faucet to allow water to flow through the condenser. Make Water out
sure the rubber tubing connections are tight and check for leaks.
9. Plug the heating mantle into the variable transformer and turn on the
power to begin heating the flask.
10. When the water in the flask boils, the vapor will rise in the distillation
Water in
appa- ratus and condense in the condenser. The liquid will collect in the
receiving flask.
11. Continue distilling the mixture for 45 minutes, until about 30 mL of distil-
late has been collected. Record the boiling point of the distillate and its
appearance in the data table.
12. Turn off the heating mantle. Allow the apparatus to cool and then
remove the receiving flask. Ice bath
13. Pour the distillate into a large test tube and add solid sodium chloride until To power
supply
the solution is saturated. Pour the liquid (not the solid) into a separatory
funnel.
Figure 2. Steam Distillation Apparatus.
14. Add about 7 mL of hexane to the separatory funnel and shake. Run off the
lower aqueous layer back into the large test tube, and collect the upper
hex- ane layer in a 125-mL Erlenmeyer flask.
15. Return the aqueous layer back into the separatory funnel, and repeat step 14. Combine the hexane extracts in the
same Erlenmeyer flask.
16. Working in the hood, place a stirring rod in the hexane solution and heat the solution on a hot plate at a medium setting.
Allow all of the hexane to evaporate.
17. (Optional) Allow the hexane to evaporate at room temperature overnight.
18. Cool the flask to room temperature. Observe the appearance of oil of cinnamon and carefully waft the vapor to your
nose to detect the odor. In the data table, record the appearance and odor of the essential oil.
19. Obtain two small test tubes and place about 1 mL of Schiff reagent into each test tube.
20. Add one drop of pure cinnamaldehyde to the first test tube and one drop of the essential oil to the second test tube.
Record observations.
Disposal
Please consult your current Flinn Scientific Catalog/Reference Manual for general guidelines and specific procedures, and review
all federal, state and local regulations that may apply, before proceeding. The reaction product may be disposed of down the
drain with cold running water according to Flinn Suggested Disposal Method #26b.
Connecting to the National Standards
This laboratory activity relates to the following National Science Education Standards (1996):
Unifying Concepts and Processes: Grades K–12
Evidence, models, and explanation
Content Standards: Grades 9–12
Content Standard B: Physical Science, structure and properties of matter
Content Standard G: History and Nature of Science, nature of scientific knowledge, historical perspectives
Tips
• This lab is best suited for an advanced or honors chemistry class or as a demonstration. There are two good stopping
points if the lab cannot be finished in the scheduled class time. After extracting the oil from the distillate, save the
hexane
extract before evaporating the solvent. Testing the oil of cinnamon will only take a few minutes. This step can easily be completed
on a second day.
• Demonstrate the proper use of a separatory funnel. Always invert the stoppered separatory funnel and vent the vapor
3 © 2016 Flinn Scientific, Inc. All Rights
Steam Distillation of Cinnamon
before shaking the funnel, and then again after every shake. Failure to vent the funnel may result in pressure building
4 © 2016 Flinn Scientific, Inc. All Rights
Steam Distillation of Cinnamon
up and solvent spraying out of the separatory funnel when the stopper is removed.
• For best results, always use fresh cinnamon sticks for steam distillation. Cinnamaldehyde and other components of
cinnamon oil are volatile liquids and may evaporate from opened packages of cinnamon.
• Schiff’s reagent is used as a qualitative test for aldehydes. It consists of an indicator dye, fuchsin hydrochloride, in a satu
rated solution of sulfur dioxide. Sulfur dioxide decolorizes the dye. When an aldehyde is added to Schiff ’s reagent, it
reacts with the SO2 and thus restores the deep reddish purple color of the dye.
• Essential oils are considered secondary metabolites. Many leaf oils and root oils are natural pesticides, protecting plants
against insects and parasites. Essential oils from flowers may help plants attract insects for pollination. Many essential oils
also have natural antifungal and antibacterial ability.
Sample Data Table (Student data will vary.)
Boiling Point = 99–100 °C. The distillate looked oily and separated
Boiling Point and Appearance of Distillate
into two layers in the receiving flask.
Oil of Cinnamon—Appearance and Odor The oil was a pale yellow liquid with a strong cinnamon odor.
Results of Schiff Test for Aldehydes
Cinnamaldehyde The sample gave a deep magenta (purple) solution after two minutes.
Oil of Cinnamon Same as above—deep purple product.
Answers to Questions (Student answers will vary.)
1. The distillate looked like oily water. It was two layers. When the liquid in the condenser collected in the receiving flask, the oil seemed to
be the bottom layer. Oil of cinnamon is thus more dense than water. Note to teachers: Most essential oils are less dense than water. Oil
of cinnamon is an exception.
2. Cinnamon oil has a stronger smell than cinnamon sticks. That is because there may be many more components of the odor of fresh natu-
ral cinnamon in addition to the essential oil. The other components modify the odor.
3. Cinnamaldehyde is a known aldehyde. It served as a positive control for the test with Schiff’s reagent. A positive test was marked by the
formation of a dark magenta (reddish purple) solution. Oil of cinnamon gave a positive test.
4. Vapor pressure of water = 760 – 5 mm Hg = 755 mm Hg.
Cinnamaldehyde, C9 H8 O, molar mass 131 g/mole
mass of cinnamaldehyde (g) P × MM (oil)
————————————— = —————————— oil
mass of water (g) PH O × MM (water)
2
x grams cinnamaldehyde (5 mm Hg)(131 g/mole)
———————————— = ————————————
30 g water (755 mm Hg)(18 g/mole)
x = 1.5 g cinnamaldehyde per 30 g of water
5. The combined vapor pressure of a mixture of two immiscible liquids is equal to the sum of their individual vapor pressures. Because a
liquid will boil when the total vapor pressure is equal to atmospheric pressure, the boiling point of the mixture will be less than the boil-
ing point of either component.
6. Essential oils are responsible for the strong odors or fragrance of plants. Odors help plants attract insects for pollination. Also, some odors
may actually repel insects that may be harmful. Note to teachers: A good example is citronellol, which is the essential oil from lemon-
grass. Citronella candles are used to keep mosquitoes away.
5 © 2016 Flinn Scientific, Inc. All Rights
Steam Distillation of Cinnamon
Materials for Steam Distillation of Cinnamon are available from Flinn Scientific, Inc.
Catalog No. Description
C0400 Cinnamaldehyde, 100-mL
H0002 Hexanes, Reagent, 500 mL
S0180 Schiff Reagent, 100 mL
S0134 Sodium Chloride, 500 g
Consult your Flinn Scientific Catalog/Reference Manual for current prices.
6 © 2016 Flinn Scientific, Inc. All Rights
Steam Distillation of Cinnamon
Steam Distillation of Cinnamon Worksheet
Data Table
Boiling Point and Appearance of Distillate
Oil of Cinnamon—Appearance and Odor
Results of Schiff Test for Aldehydes
Cinnamaldehyde
Oil of Cinnamon
Questions (Use a separate sheet of paper to answer the following questions.)
1. Describe the appearance of the distillate. Is cinnamaldehyde (oil of cinnamon) less dense or more dense than water?
2. Compare the odor of cinnamon oil versus cinnamon sticks. What factors may account for the difference in smell?
3. Schiff’s reagent is used as a qualitative test for aldehydes. What compound was used as a positive control or reference
com- pound for this test? Describe the appearance of a positive test result. Did oil of cinnamon give a positive test?
4. The vapor pressure of cinnamaldehyde at 99–100°C is about 5 mm Hg. Assuming that the barometric pressure was 760
mm Hg, use Equations 1 and 3 in the Background section to calculate the mass of cinnamon oil that would co-distill with
30 g of water during steam distillation.
5. Why is the boiling point of a mixture of an essential oil and water always less than 100 °C?
6. Essential oils and other natural products are considered secondary metabolites—they are not responsible for the primary
structure and function of a cell. What are some possible biochemical functions for an essential oil?
7 © 2016 Flinn Scientific, Inc. All Rights
You might also like
- Steam PLDocument12 pagesSteam PLPia JoNo ratings yet
- Steam Distillation of Cloves READ:Landgrebe 4 Ed .p160-161 (Section 7.6) and p369-371, 5 Ed. P 140-141 (Section 7.6) and P 321-322Document4 pagesSteam Distillation of Cloves READ:Landgrebe 4 Ed .p160-161 (Section 7.6) and p369-371, 5 Ed. P 140-141 (Section 7.6) and P 321-322AeFondevillaNo ratings yet
- Determination of Aniline Point of Petroleum SamplesDocument4 pagesDetermination of Aniline Point of Petroleum SamplesJay RanjanNo ratings yet
- W W W Rhodium W S Hemistry RchiveDocument2 pagesW W W Rhodium W S Hemistry RchiveMirza CaticNo ratings yet
- Chem 2 ExperimentsDocument5 pagesChem 2 ExperimentsSKNo ratings yet
- Stereo ChemistryDocument4 pagesStereo ChemistryDORINA MANTUNo ratings yet
- Laporan DESTILASI FRAKSINASIDocument12 pagesLaporan DESTILASI FRAKSINASIYunita RachmawatiNo ratings yet
- CH (CO H), Pyr, Heat K CO Water or AcetoneDocument4 pagesCH (CO H), Pyr, Heat K CO Water or AcetoneAlexander Niño CarvajalNo ratings yet
- Chemistry 40 Synthesis of Aspirin (Please Edit Schematic Diagram)Document6 pagesChemistry 40 Synthesis of Aspirin (Please Edit Schematic Diagram)Jayme Paolo100% (1)
- Phenolic Resin PreparationDocument7 pagesPhenolic Resin PreparationmitaasthaNo ratings yet
- Essential Oils PDFDocument3 pagesEssential Oils PDFJonathan WongNo ratings yet
- Methylamine RecipeDocument4 pagesMethylamine RecipeVanilla470% (1)
- CHEM 230 - Lecture 5 - 2021 Steam DistillationDocument19 pagesCHEM 230 - Lecture 5 - 2021 Steam DistillationAhmed AliNo ratings yet
- Experiment 3: Esterifications Reactions of Vanillin: The Use of NMR To Determine A StructureDocument19 pagesExperiment 3: Esterifications Reactions of Vanillin: The Use of NMR To Determine A StructureDang Humairah100% (1)
- Tabla de Colores PDFDocument21 pagesTabla de Colores PDFSilvio Latini SpahnNo ratings yet
- Lab 9 - Cinnamaldehyde LabDocument4 pagesLab 9 - Cinnamaldehyde LabErika Montilla100% (1)
- H N O 1. H O (100 C) 2. Decolorizing Carbon 3. Ice Bath (0 C) H N ODocument5 pagesH N O 1. H O (100 C) 2. Decolorizing Carbon 3. Ice Bath (0 C) H N OFriendlee Zoe GacutnoNo ratings yet
- Reading Assignment: Mohrig Sections 14 - 15 (Melting Point & Recrystallization)Document5 pagesReading Assignment: Mohrig Sections 14 - 15 (Melting Point & Recrystallization)Mirza MohammadNo ratings yet
- Balmer Exp 6Document6 pagesBalmer Exp 6Collin ChuNo ratings yet
- 2423L4Document8 pages2423L4unsaniaNo ratings yet
- Miscellaneous ChemicalsDocument8 pagesMiscellaneous Chemicalsgbonger100% (8)
- PA-PAC Eutectic MixturesDocument4 pagesPA-PAC Eutectic MixturesRajeshNo ratings yet
- Lecture 2Document24 pagesLecture 210A 1 ANSH PANARANo ratings yet
- Preparation of Asperine and Oil of WintergreenDocument4 pagesPreparation of Asperine and Oil of WintergreenKaye ArcherNo ratings yet
- (2A) Liquid FuelsDocument26 pages(2A) Liquid FuelsJadin Zam DoctoleroNo ratings yet
- EsterificationDocument5 pagesEsterificationJoonsuk LeeNo ratings yet
- Lab 16 Hess's LawDocument19 pagesLab 16 Hess's Lawjohn linNo ratings yet
- Preparation of Diethyl EtherDocument5 pagesPreparation of Diethyl Etherjiskate77100% (2)
- 2410 Lab Cinnamon W-Br2 TestDocument2 pages2410 Lab Cinnamon W-Br2 TestErika MontillaNo ratings yet
- جداول Mass for ResinDocument15 pagesجداول Mass for ResinDl Al-azizNo ratings yet
- How To Test Your Lime Cao Available 0Document2 pagesHow To Test Your Lime Cao Available 0cassindromeNo ratings yet
- BL Nurbio Activity 4 - Lipids (Revised 6.22.20) - EditedDocument8 pagesBL Nurbio Activity 4 - Lipids (Revised 6.22.20) - EditedDouble DeckerNo ratings yet
- Perrys Table 2-2Document21 pagesPerrys Table 2-2martha suriNo ratings yet
- CHEM F110 - Lab Manual - Nov 5-2020Document45 pagesCHEM F110 - Lab Manual - Nov 5-2020STUTI MATHUR100% (2)
- Section-B: A Practical Book of Medicinal Chemistry-I (Sem. - V)Document19 pagesSection-B: A Practical Book of Medicinal Chemistry-I (Sem. - V)Raju NiraulaNo ratings yet
- Recrystallization of Benzoic Acid PDFDocument7 pagesRecrystallization of Benzoic Acid PDFericaNo ratings yet
- Method To Determine The Saponification Value of Tall Oil: PCTM 16Document1 pageMethod To Determine The Saponification Value of Tall Oil: PCTM 16Vinay KumarNo ratings yet
- Exp 37 Friedel Crafts Supplemental ReadingDocument9 pagesExp 37 Friedel Crafts Supplemental Readingmayagal1707No ratings yet
- Experiments - LBYCH34 Experiment Manual Student SDocument24 pagesExperiments - LBYCH34 Experiment Manual Student SShean Berwin GonzalesNo ratings yet
- Chem 361 Lab 4Document5 pagesChem 361 Lab 4Daood Tanvir GillNo ratings yet
- Physpharm PrefiDocument10 pagesPhyspharm PrefiErich ElloNo ratings yet
- Methods of PuriDocument10 pagesMethods of PuriZirwaNo ratings yet
- 1T 19-20 LBYKM31 Experiment Manual As of Sept 15 2019Document33 pages1T 19-20 LBYKM31 Experiment Manual As of Sept 15 2019Ammi PinedaNo ratings yet
- 2C H Via 2,5 Dimethoxymandelonitrile2Document1 page2C H Via 2,5 Dimethoxymandelonitrile2Fermin GamboaNo ratings yet
- DistillationDocument12 pagesDistillationAbhishek SardaNo ratings yet
- General Formula Alkane C: AlkanesDocument13 pagesGeneral Formula Alkane C: AlkanesPedro Moreno de SouzaNo ratings yet
- PFRDocument1 pagePFRJocelyn Grisel García GonzálezNo ratings yet
- 43.1.14 AOAC of Fi Cial Method 962.17 Vol A Tile Oil in SpicesDocument2 pages43.1.14 AOAC of Fi Cial Method 962.17 Vol A Tile Oil in SpicespiagiopersempreNo ratings yet
- Ab-141 4 en PDFDocument17 pagesAb-141 4 en PDFAfthirah AmiraNo ratings yet
- Distillation of Binary LiquidsDocument29 pagesDistillation of Binary LiquidsJan Lloyd ProbitsoNo ratings yet
- Experiment 1 Distillation of Tanduay ComponentsDocument8 pagesExperiment 1 Distillation of Tanduay ComponentsFritzie JumawidNo ratings yet
- New Sources of 9-D-Hydroxy-cis-12-octadecenoic Acid: L AnderDocument4 pagesNew Sources of 9-D-Hydroxy-cis-12-octadecenoic Acid: L AnderRaja LakshmiNo ratings yet
- Xanthan GumDocument2 pagesXanthan GumKasidit SornchaiNo ratings yet
- Assay of Volatile Oils, Ethereal OilsDocument25 pagesAssay of Volatile Oils, Ethereal OilshazelNo ratings yet
- H/ H Ratio Analysis of Flavor Compounds by On-Line Gas Chromatography-Pyrolysis-Isotope Ratio Mass Spectrometry (HRGC-P-IRMS) : CitralDocument5 pagesH/ H Ratio Analysis of Flavor Compounds by On-Line Gas Chromatography-Pyrolysis-Isotope Ratio Mass Spectrometry (HRGC-P-IRMS) : CitralРусланNo ratings yet
- A Concise Text-Book of Organic Chemistry: The Commonwealth and International Library: Chemistry DivisionFrom EverandA Concise Text-Book of Organic Chemistry: The Commonwealth and International Library: Chemistry DivisionRating: 5 out of 5 stars5/5 (2)
- Advanced Pharmaceutical analysisFrom EverandAdvanced Pharmaceutical analysisRating: 4.5 out of 5 stars4.5/5 (2)
- Schaum's Easy Outline of Organic Chemistry, Second EditionFrom EverandSchaum's Easy Outline of Organic Chemistry, Second EditionRating: 3.5 out of 5 stars3.5/5 (2)
- Essential Oil Chemistry Formulating Essential Oil Blends that Heal - Aldehyde - Ketone - Lactone: Healing with Essential OilFrom EverandEssential Oil Chemistry Formulating Essential Oil Blends that Heal - Aldehyde - Ketone - Lactone: Healing with Essential OilRating: 5 out of 5 stars5/5 (1)
- The Chemistry of Fertilisers and Manure - Including Information on the Chemical Constituents and Types of Fertilisers and ManuresFrom EverandThe Chemistry of Fertilisers and Manure - Including Information on the Chemical Constituents and Types of Fertilisers and ManuresRating: 5 out of 5 stars5/5 (1)