Professional Documents
Culture Documents
Alkyl Halides DPP2
Uploaded by
gamerion2006Original Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Alkyl Halides DPP2
Uploaded by
gamerion2006Copyright:
Available Formats
DPP - Daily Practice Problems
Name : Date : ��------�
Start Time : End Time :
I
CHEMISTRY
SYLLABUS : Haloalkanes and Haloarenes-11 :
[ 4
Properties and Usesof Halogen Containing Compounds7]
Max. Marks : 120 Time : 60 min.
GENERAL INSTRUCTIONS
•
The Daily Practice Problem Sheet contains 30 MCQ's. For each question only one option is correct. Darken the correct drcle/
bubble in the Response Grid provided on each page.
•
You have to evaluate your Response Grids yourself with the help of solution booklet.
•
Each correct answer will get you 4 marks and 1 mark shall be deduced for each incorrect answer. No mark will be given/ deducted
if no bubble is filled. Keep a timer in front of you and stop immediately at the end of 60 min.
• The sheet follows a particular syllabus. Do not attempt the sheet before you have completed your preparation for that syllabus.
Refer syllabus sheet in the starting of the book for the syllabus of all the DPP sheets.
• After completing the sheet check your answers with the solution booklet and complete the Result Grid. Finally spend time to
analyse your performance and revise the areas which emerge out as weak in your evaluation.
DIRECTIONS (Q.1-Q.21) : There are 21 multiple choice Q.3 Reaction of ter-butyl br·omide with sodium methoxide
questions. Each question has 4 choices (a), (b), (c) and (d), produces
out of which ONLY ONE choice is correct. (a) isobutane
Q.l Treatment of ammonia with excess of ethyl chl ori de will (b) isob utylene
yield (c) sodium- ter-butoxide
(a) diethyl amine (d) /er-butyl methyl ether
(b) ethane Q.4 Arrange tJ1e following compounds in order of increasing
(c) tetraethyl ammonium chloride dipole moment :
(d) methyl amine toluene (I),
Q.2 Chlorobenzene is m-dichlorobenzene (ll),
(a) less reactive than benzyl chloride a-dichlorobenzene (lll),
(b) more reactive than ethyl bromide p-dichlorobenzene (IV)
(c) n earl y as reactive as methyl chloride (a) I < IV< ll < III (b) IV< 1 < II < Ill
(d) more reactive than isopropyl chloride (c) IV < I < III<II (d) IV < l i < I < lll
1. ®®@@ 2. ®®@@ 3. ®®@@ 4. ®®@@
------ Spacefor Rough Work ------
CC14 cannot give precipitate with AgN03 due to Q.12 The major product obtained on treatment of
(a) formation ofcomplex withAgN03 CH3C�CH(F)CH3 with CH30-/CH30His
(b) evolution ofC1 2 gas (a) CH3C�CH(OC�)CH3 (b) CH3CH= CHCII:3
(c) chloride ion is not formed (c) CH3C�CH=C� (d) CH3CR:zC�C�OCH3
(d) AgN03 does not give silver ion
�
Q.13 Identify the products A and B in the following reaction.
Q.6 Ethyl bromide reacts with silver nitrite to form
hv V
HBr HBr
(a) Nitroethane B A
if
(b) Nitroethane and ethyl nitrite Br
(c) Ethyl nitrite
(d) Ethane (a) Both A and B acc
�
Q.7 Chlorobenzene on fusing with solid NaOH gives
(a) Benzene (b) Benzoicacid
(c) Phenol (d) Benzene chloride (b) Both Aand B are r
Q.8 A compound (A) bas a molecular formula C2CI30H. It
if �
Br
reduces Fehling solution and on oxidation gives a
monocarboxylic acid (B). (A) is obtained by action of (c) A is & B is '
chlorine on ethyl alcohoL (A) is
� if
(a) Chloral (b) CHCI3 Br
(c) CH3 CI (d) Chloroacetic acid
(d) Ais < & Bis
Q.9 A sample of chloroform being used as anaesthetic is tested
by
OH
(a) Fehling solution I
(b) Ammoniacal Cu2 Cl2 Q.14 The comp01md (CH3)2 - C -CCI3 is
(c) AgN03 solution after boiling with alcoholic KOH (a) Cbloretone
solution (b) Chloroquine
(d) none of these (c) Chloropicrin
Q.lO Among the following, the one which reacts most readily (d) Chloropropyl chloride
with ethanol is
(a) p- nitrobenzyl bromide
Q.15 The fire extinguisher, pyrene is
(a) C02 (b) CCI4 (c) CS2 (d) CHCJ3
(b) p- ch lorobenzyl bromide Q.16 B.H.C is used as
(c) p-methoxybenzyl bromide (a) insecticide (b) pesticide
(d) p- methylbenzyl bromide (c) herbicide (d) weedicide
Q.ll Two percent of ethanol is added during the oxidation of Q.17 An organic halide is shaken with aqueous NaOH followed
chloroform to stop the formation of carbonyl chloride. by the addition ofdil. HN03 and silver nitrate solution and
In this reaction ethanol acts as gives white ppt. The substance can be
(a) auto catalyst (b) negative catalyst (a) C6H4 (CH3)Br (b) C6H5C�Cl
(c) positive catalyst (d) none of these (c) C6H5Cl (d) None of these
5. ®®0@ 6. ®®0@ 7. ®®0@ 8. ®®@@ 9. ®®0@
10.®®0@ 11. ®®0@ 12.®®0@ 13.@®@@ 14. ®®0@
15.@@0@ 16. @®0@ 17.®®0@
------- Space for Rcugh Work -------
DPP/ C ( 4 7 ) ------------------..... 187
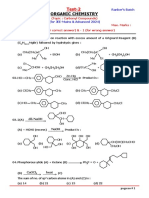
Q.18 Which plastic is obtained from CHCI3 as follows ? Q.23 Which ofthe following statements about benzyl chloride
HF 800 y
0
are correct ?
CHCI3 Sbf.J
X
C Polymerisation
Plastic (l) It can be oxidised to benzaldehyde by boiling with
copper nitrate solution
(a) Bakelite (b) Teflon (c) Polytbene (d) Perspex
test
(2) It is a lachrymatory liquid and answers Beilstein's
Q.19 Which one of the following is the correct formula of
dichlorodiphenyl trichloroethane (3) I t gives a white precipitate with alcoholic silver
'
H CJ
___;-;::'\___ I I
'
H Cl
6
�I I
6
nitrate.
(a) c1� c- c- CJ � C- C- CI ( 4) It is less reactive than alkyl halides
@
(b)
$
Q.24 A mixture oftwo organic chlorine compounds was treated
with sodium metal in ether solution. Isobutane was ob
tained as a product. The two chlorine compounds are
H
Cl ( I ) Methyl chloride (2) Ethyl chloride
'
___/"";;\_ I I
Cl Cl
___;-;::'\___ I
Cl
�C- C - CI
�
(d) C J � C - C - C I
I (3) Isopropyl chloride (4) Propyl chloride
(c) CI
@i' $
DIRECTION (Q.25-Q.27) : Read the passage given below
and answer the questions that follows :
Grignard reagents (RMgX) are prepared by the reaction of an
Cl
Q.20 The set of compounds in which the reactivity of halogen organic halide and magnesium metal in ether solvent.
R - X + Mg
atom in the ascending order is R-O-R
R - MgX
(a) Vinyl chloride, chloroethane, chlorobenzene
The solvent (usually diethyl ether or tetrahydrofuran) plays a
(b) Vinyl chloride, chlorobenzene, chloroethane
(c) Chloroethane, chlorobenzene, vinyl chloride cmcial role in the formation ofGrignard reagent. Alkyl halides
(d) Chlorobenzene, vinyl chloride, chloroethane are more reactive than aryl and vinyl halides. Indeed, aryl and
vinyl chlorides do not form Grignard reagents in dietJ1yl ether.
However, an alkyl halide containing an alcoholic - OH group is
Q.21 Which of the following is boiled with ethyl chloride to
form ethyl alcohol ?
converted to Grignard reagent by first protecting the --OH group
with tert-butyldimethylsilyl ether which is inert to Grignard reagent.
(a) Alcoholic KOH (b) Aqueous KOH
(c) �0 (d) �02
The protecting group is finally liberated by treatment with fluoride
DIRECTIONS (Q.22-Q.24) : In the following questions, lOll.
more than one of the answers given are correct. Select
the correct answers and mark it according to the following
codes:
Codes :
(a) I , 2 and 3 are correct (b) l and 2 are correct
tert-Butylchloro:limethylsilane
(c) 2 and 4 are correct (d) l and 3 are correct
Q.22 Freon is not used
(I) as local anaesthetic
(2) for dissolving impurities in metallurgical process
(3) in printing industry
(4) in refrigerator
R�.SI'O:\SE 18.®®0@ 19. ®®0@ 20. ®®0@ 21. ®®@@ 22. ®®0@
GRm 23.®®0@ 24.®®0@
------ Spacefor Rough Work ------
,...._
......
I{)
,...._
I
0
188 1--- DPP/ C ( 4 7 )
(!]
w
Q.25 Grignard reactions generally occur in dry ether because DIRECTIONS (Q. 28-Q.30) : Each ofthese questions contains
(a) The stronger acid diethyl ether will displace the
weaker RH acid from its salt.
two statements: Statement-! (Assertion) and Statement-2
(Reason). Each ofthese questions has four alternative choices,
(b) The stronger acid �0 will displace the weaker acid only one of which is the correct answer. You have to select the
RH from its salt correct choice.
(c) Wat·er slows down the reaction
(a) Statement-! is True, Statement-2 is True; Statement-2 is
(d) Water mixes with ether preventing ether to perform
a correct explanation for Statement-I .
its function.
(b) Statement-! is True, Statement-2 is True; Statement-2 is
Q.26 Grignard reagent can't be prepared from
NOT a correct explanation for Statement-1.
©
(c) Statement -1 is False, Statement-2 is True.
Cl
(d) Statement -1 is True, Statement-2 is False.
(a) HO �sr (b) Q.28 Statement-I :Aqueous bydrohalogen acids are used to
� Cl prepare alkyl halides from alkenes.
Statement-2 :Hydrogen iodide readily reacts with alkenes
f-.-ci
Cl
to form alkyl halides.
(c) (d)
Q.29 Statement -1 : Electron wi thdrawiog groups in aryl halides
Q.27 The function of tetrahydrofuran in the preparation of increase the reactivity towards nucleophilk substitution.
Grignard reagent is that it Statement -2 : 2, 4-Dioitrochlorobenzene is less reactive
(a) acts as a solvent than chlorobenzene.
(b) helps in maintaining the reactivity of magnesium Q.30 Statement - 1 : Optically active 2-iodobutane on treatment
(c) both with Nal in acetone undergoes racemization.
(d) none of the two Statement-2 : Repeated Walden inversions on the reactant
and its product eventually gives a racemic mixture.
R�:;po:-;s�. 25.®®0@ 26.®®0@ 27.®®0@ 28.®®®@ 29. ®®0@
GRill 30. ®@@@
DAILY P RACTICE PROBLEM SHEET 47 - CHEMISTRY
Tota l Questions 30 Tota l Marks 120
Attempted Correct
Incorrect Net Score
Cut-off Score 36 Qual ifying Score 60
Success Gap = Net Score - Qual ifying Score
N et Score = (Correct x 4} - ( Incorrect x 1)
------- Space for Rcugh Work -------
You might also like
- Carbonyl Compounds Xi Xii Study MaterialsDocument171 pagesCarbonyl Compounds Xi Xii Study MaterialsCristiano Hamdiansyah SempadianNo ratings yet
- Class Test-1-Aldehydes & Ketones - PreparationDocument5 pagesClass Test-1-Aldehydes & Ketones - PreparationSarthak VermaNo ratings yet
- Topic Page No. Nomenclature: Organic ChemistryDocument22 pagesTopic Page No. Nomenclature: Organic ChemistryRishabh Sharda100% (1)
- Hydrocarbons MCQDocument5 pagesHydrocarbons MCQTukun KunuNo ratings yet
- Organic Chemistry Class 11 Notes by Bharat PanchalDocument24 pagesOrganic Chemistry Class 11 Notes by Bharat Panchalsaurabh Kumar78% (9)
- Aldehydes, Ketones and Carboxylic Acids - Practice SheetDocument4 pagesAldehydes, Ketones and Carboxylic Acids - Practice Sheetsameeryad72No ratings yet
- Alcohol & Ether B Lord of The RingsDocument13 pagesAlcohol & Ether B Lord of The RingsadityaNo ratings yet
- FOSFA Banned List PDFDocument2 pagesFOSFA Banned List PDFanggirasti0% (2)
- Aromatic CompoundsDocument16 pagesAromatic CompoundsadityaNo ratings yet
- Index: Hydrocarbons (Alkanes, Alkenes & Alkynes)Document31 pagesIndex: Hydrocarbons (Alkanes, Alkenes & Alkynes)Harsh VardhanNo ratings yet
- Alkyl Halides DPP1Document4 pagesAlkyl Halides DPP1gamerion2006No ratings yet
- 06 - Toluene (Level) Module-4Document10 pages06 - Toluene (Level) Module-4Raju SinghNo ratings yet
- 13 DPP 09a-09d Halk & Harn EvolveDocument17 pages13 DPP 09a-09d Halk & Harn Evolvemangeshchavan980No ratings yet
- Carbonyl Compounds 12thDocument24 pagesCarbonyl Compounds 12thRaju SinghNo ratings yet
- Chemistry Combine Alkyl HalideDocument18 pagesChemistry Combine Alkyl HalideVanshika LudhaniNo ratings yet
- BB YASMt DVEvy ZZi PR NYRDocument8 pagesBB YASMt DVEvy ZZi PR NYRarindamNo ratings yet
- Haloalkanes and Haloarenes, Alcohols, Phenols and Ethers-31-OctDocument7 pagesHaloalkanes and Haloarenes, Alcohols, Phenols and Ethers-31-Octolivia.benson9331No ratings yet
- Amines & Diazonium Salt - Practice SheetDocument11 pagesAmines & Diazonium Salt - Practice Sheetdptrtfn879No ratings yet
- Hydrocar SHEET3Document4 pagesHydrocar SHEET3Aayush SaxenaNo ratings yet
- 655f0ac18d579400185b427d - ## - Aromatic Compounds Questions Notes Lakshya JEE 2.0 2024Document58 pages655f0ac18d579400185b427d - ## - Aromatic Compounds Questions Notes Lakshya JEE 2.0 2024sontygaming001No ratings yet
- Organic Questions 1Document3 pagesOrganic Questions 1SABARI SRINIVAS ANo ratings yet
- Alkanes - Alkenes - Alkynes - DPP 3Document3 pagesAlkanes - Alkenes - Alkynes - DPP 3Vishal_93100% (1)
- Aldehydes and Ketones - 3Document6 pagesAldehydes and Ketones - 3iitlectureNo ratings yet
- ND SPL Test Xii Che Neet 15-12-23Document7 pagesND SPL Test Xii Che Neet 15-12-23Deena chemistNo ratings yet
- Halogen Derivative of Alkanes & Arenes (Level - Iii & Iv) : Choh Socl CHCL So HCLDocument4 pagesHalogen Derivative of Alkanes & Arenes (Level - Iii & Iv) : Choh Socl CHCL So HCLAbhi WanwadeNo ratings yet
- Exersice PDFDocument24 pagesExersice PDFharsh mishraNo ratings yet
- VDA - 8 Carbon and Its CompoundDocument5 pagesVDA - 8 Carbon and Its CompoundArpit AgarwalNo ratings yet
- Amines MCQDocument3 pagesAmines MCQaleena'No ratings yet
- 03 - Acid Derivatives (Level) Module-5Document14 pages03 - Acid Derivatives (Level) Module-5Raju SinghNo ratings yet
- MCQ Chemistry Practice Qwestions Class 12thDocument8 pagesMCQ Chemistry Practice Qwestions Class 12thMithun ChakladarNo ratings yet
- Halo Alkanes Sample PaperDocument6 pagesHalo Alkanes Sample PapervasuNo ratings yet
- 3B-HYDROCARBON Assignment - FinalDocument49 pages3B-HYDROCARBON Assignment - Finalkraken monsterNo ratings yet
- Aromatic Compounds (13th)Document24 pagesAromatic Compounds (13th)Raju SinghNo ratings yet
- 6 - QP and MS - Haloalkanes and HaloarenesDocument9 pages6 - QP and MS - Haloalkanes and Haloareneskrish dabhi0% (1)
- Kcet - Chemistry - 2019: Version Code: D-5Document7 pagesKcet - Chemistry - 2019: Version Code: D-5Manoj CNo ratings yet
- New Chaptest - Hydrocarbons For KKTYR02A01, KKTYW02F01 BatchDocument23 pagesNew Chaptest - Hydrocarbons For KKTYR02A01, KKTYW02F01 Batchiamxxxofficial86No ratings yet
- Topic: Organic Compounds Containing HalogensDocument5 pagesTopic: Organic Compounds Containing Halogensvictoria schoolNo ratings yet
- Quiz Organic 1Document6 pagesQuiz Organic 1ronakgupta332005No ratings yet
- Pdf&rendition 1Document11 pagesPdf&rendition 1Ishita AgarwalNo ratings yet
- PDF Alkanepdf DLDocument8 pagesPDF Alkanepdf DLGeraldineNo ratings yet
- 05 - Benzoic Acid (Level) Module-5Document10 pages05 - Benzoic Acid (Level) Module-5Raju SinghNo ratings yet
- PhenolDocument6 pagesPhenoldevender singhNo ratings yet
- Organic Chemistry Test-1 On Total Syllabus: Single CorrectDocument5 pagesOrganic Chemistry Test-1 On Total Syllabus: Single CorrectVanshaj GuptaNo ratings yet
- MCQ Halo Alkanes and ArenesDocument27 pagesMCQ Halo Alkanes and ArenessarahNo ratings yet
- Alkyl and Aryl Halides SheetDocument11 pagesAlkyl and Aryl Halides SheetRajeev GangwarNo ratings yet
- Time: 1 Hrs Max. Marks: 98 Single Correct: 3 2 4 HG ZNDocument6 pagesTime: 1 Hrs Max. Marks: 98 Single Correct: 3 2 4 HG ZNlakshmi.vedanarayanan7785No ratings yet
- Wa0022.Document4 pagesWa0022.michaeldcosta414No ratings yet
- Chemistry 2nd Year Eamcet Named Reaction Identification of Functional Group-1Document7 pagesChemistry 2nd Year Eamcet Named Reaction Identification of Functional Group-1Surya Charan Reddy100% (1)
- Multiple Choice Questions: Question Bank Class: Xii, Chemistry Unit 4: Haloalknaes & HaloarenesDocument27 pagesMultiple Choice Questions: Question Bank Class: Xii, Chemistry Unit 4: Haloalknaes & HaloarenesAkshita BoroNo ratings yet
- Amines - Practice SheetDocument4 pagesAmines - Practice Sheetsameeryad72No ratings yet
- Nitrogen CompoundsDocument11 pagesNitrogen CompoundsJatindra PatelNo ratings yet
- Bottom of Pyramid - Test # 15 - Aldehydes, Ketones & Carboxylic AcidsDocument7 pagesBottom of Pyramid - Test # 15 - Aldehydes, Ketones & Carboxylic AcidsJay PatelNo ratings yet
- Chem CGRDocument5 pagesChem CGRpinnaacleclasses salemNo ratings yet
- ChemistryDocument7 pagesChemistryrjakrithiNo ratings yet
- Alcohols Phenols and Ether - DPP - 4Document3 pagesAlcohols Phenols and Ether - DPP - 4Priya RangapureNo ratings yet
- Home Assignment-3Document32 pagesHome Assignment-3ansh guptaNo ratings yet
- Alkynes 1Document3 pagesAlkynes 1Anonymous vRpzQ2BL100% (1)
- NEET - Halo Alkanes and Halo Arenes Practice PaperDocument3 pagesNEET - Halo Alkanes and Halo Arenes Practice PaperGanga DharaNo ratings yet
- Aldehyde Ketone and Carboxylic AcidDocument3 pagesAldehyde Ketone and Carboxylic Acidsonidhruv2206No ratings yet
- 01 - Carbonyl Compound (Aldehyde & Ketone) (Level) Module-5Document19 pages01 - Carbonyl Compound (Aldehyde & Ketone) (Level) Module-5Raju SinghNo ratings yet
- Haloalkanes and HaloarenesDocument18 pagesHaloalkanes and HaloarenesBhavesh KNo ratings yet
- Term-1 Practice Test (Complete Syllabus) : Sample PaperDocument6 pagesTerm-1 Practice Test (Complete Syllabus) : Sample PaperDarshan NayakNo ratings yet
- Kinetic Theory of GasesDocument201 pagesKinetic Theory of Gasesgamerion2006No ratings yet
- Thermodynamics NOTES 1Document39 pagesThermodynamics NOTES 1gamerion2006No ratings yet
- Alkenes Part 1Document25 pagesAlkenes Part 1gamerion2006No ratings yet
- 11th Functions 3Document16 pages11th Functions 3gamerion2006No ratings yet
- Benzene and Aromaticity: Based On Mcmurry'S Organic Chemistry, 6 EditionDocument72 pagesBenzene and Aromaticity: Based On Mcmurry'S Organic Chemistry, 6 EditionAdzimahNo ratings yet
- 1st Year 2nd Sem AuxilliumDocument20 pages1st Year 2nd Sem Auxillium杨俊熙(Stanley)No ratings yet
- Biochemistry of CarbohydratesDocument7 pagesBiochemistry of CarbohydratesRobin TolentinoNo ratings yet
- Revision Sheet HaloalkaneDocument3 pagesRevision Sheet HaloalkaneSahil PandeyNo ratings yet
- Glandz Product ListDocument24 pagesGlandz Product ListRahul PambharNo ratings yet
- (L4) Carbon and Its Compounds Class10 PDFDocument24 pages(L4) Carbon and Its Compounds Class10 PDFRekha MishraNo ratings yet
- Chapter 6. Reactions of AlkynesDocument37 pagesChapter 6. Reactions of Alkynesthanhnguyenhhvn100% (1)
- EXP 5 Lab Report. Analysis of CarbohydratesDocument3 pagesEXP 5 Lab Report. Analysis of CarbohydratesAdrian Alvinson NazarenoNo ratings yet
- 1.1 - 1.3 Alkanes, Enes, Ynes, AromaticsDocument44 pages1.1 - 1.3 Alkanes, Enes, Ynes, AromaticsTiwanka MadugalleNo ratings yet
- Surface Tension of Various Liquids PDFDocument43 pagesSurface Tension of Various Liquids PDFneha sahuNo ratings yet
- Chemical Data Reporting List AlphabeticalDocument98 pagesChemical Data Reporting List AlphabeticalRosa HerreraNo ratings yet
- L10 - Introduction OF Organic CHEMISTRY and Fundamental OF Polymer Chemistry Part IDocument74 pagesL10 - Introduction OF Organic CHEMISTRY and Fundamental OF Polymer Chemistry Part IMiraNo ratings yet
- Shendy Rulida SCIENCE 9 - Alkanes, Alkenes, and Alkynes (Part 1)Document4 pagesShendy Rulida SCIENCE 9 - Alkanes, Alkenes, and Alkynes (Part 1)Shendy RulidaNo ratings yet
- Named Reactions (ORGANIC) - CHEMISTRYDocument13 pagesNamed Reactions (ORGANIC) - CHEMISTRYbaluduvamsi2000No ratings yet
- Aliphatic XIIDocument45 pagesAliphatic XIISUYOG K.C.No ratings yet
- Appendix Kimia FisikaDocument41 pagesAppendix Kimia FisikaPieter SchmidtNo ratings yet
- Pka Table PDFDocument1 pagePka Table PDFVictoria OgdenNo ratings yet
- Reaction With Miscellaneous-NPTEL PDFDocument25 pagesReaction With Miscellaneous-NPTEL PDFRathinNo ratings yet
- Organic Chemistry Ii CHM 301: (Chapter 4)Document14 pagesOrganic Chemistry Ii CHM 301: (Chapter 4)WAN NUR AISYAH WAN AZIZANNo ratings yet
- Chanel No 5 GCMSDocument4 pagesChanel No 5 GCMSabdelcreamNo ratings yet
- Chapter 2 Organic Chemistry Klein 3rd EditionDocument2 pagesChapter 2 Organic Chemistry Klein 3rd EditionJim Xie100% (1)
- KJB Answersheet Dpa-7 Goc Class 11Document3 pagesKJB Answersheet Dpa-7 Goc Class 11Gaurav KuntalNo ratings yet
- Substitution ReactionDocument1 pageSubstitution ReactionAbhishek YadavNo ratings yet
- Organic Reagents: 1. Alcoholic KOH 2. Aluminium EthoxideDocument9 pagesOrganic Reagents: 1. Alcoholic KOH 2. Aluminium EthoxideAarya Nandal100% (1)
- Intro To Org ChemDocument21 pagesIntro To Org ChemJulius Memeg PanayoNo ratings yet