Professional Documents
Culture Documents
Memo On The Example in Unit 7C Note
Uploaded by
Mbali Mdlalose0 ratings0% found this document useful (0 votes)
10 views1 pageA sample weighing 0.1342 g of Na2S2O3 was diluted and placed in an electrochemical cell along with potassium iodide, phosphate buffer, and starch indicator. Electrolysis was carried out at 36.45 mA and took 221.8 seconds to reach the endpoint, as indicated by the starch changing color. The purity of the Na2S2O3 sample can be determined using the measured current and time along with the mole of electrons transferred in the reaction.
Original Description:
practice question
Original Title
MEMO ON THE EXAMPLE IN UNIT 7C NOTE
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentA sample weighing 0.1342 g of Na2S2O3 was diluted and placed in an electrochemical cell along with potassium iodide, phosphate buffer, and starch indicator. Electrolysis was carried out at 36.45 mA and took 221.8 seconds to reach the endpoint, as indicated by the starch changing color. The purity of the Na2S2O3 sample can be determined using the measured current and time along with the mole of electrons transferred in the reaction.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
10 views1 pageMemo On The Example in Unit 7C Note
Uploaded by
Mbali MdlaloseA sample weighing 0.1342 g of Na2S2O3 was diluted and placed in an electrochemical cell along with potassium iodide, phosphate buffer, and starch indicator. Electrolysis was carried out at 36.45 mA and took 221.8 seconds to reach the endpoint, as indicated by the starch changing color. The purity of the Na2S2O3 sample can be determined using the measured current and time along with the mole of electrons transferred in the reaction.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 1
MEMO ON THE EXAMPLE IN UNIT 7C NOTE
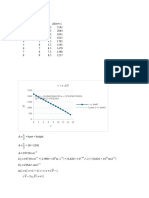
To determine the purity of a sample of Na2S2O3 , a sample is titrated coulometrically using I – as a
mediator and I3 – as the titrant. A sample weighing 0.1342 g is transferred to a 100-mL volumetric flask
and diluted to volume with distilled water. A 10.00-mL portion is transferred to an electrochemical
cell along with 25 mL of 1 M KI, 75 mL of a pH 7.0 phosphate buffer, and several drops of a starch
indicator solution. Electrolysis at a constant current of 36.45 mA requires 221.8 s to reach the starch
indicator endpoint. Determine the sample’s purity.
Number of electron is 1 ; n = 1
You might also like
- Practice Problems - Double Indicator, Precipitation, ComplexDocument3 pagesPractice Problems - Double Indicator, Precipitation, ComplexKwien AustriaNo ratings yet
- Water Analysis PrtocolDocument29 pagesWater Analysis PrtocolRajSharmaNo ratings yet
- Soal-Soal Polarografi VoltammetriDocument4 pagesSoal-Soal Polarografi VoltammetriFauzan FajariNo ratings yet
- Experiment No. 3 Application of Titrimetric Analysis: ObjectivesDocument2 pagesExperiment No. 3 Application of Titrimetric Analysis: ObjectivesIanaNo ratings yet
- Electro GravimetryDocument3 pagesElectro GravimetryUswatul ChasanahNo ratings yet
- Lab ManualDocument19 pagesLab Manualanon_467104036No ratings yet
- Soal AnalitikDocument3 pagesSoal AnalitikNurlaeli NaelulmunaMajdiyah0% (1)
- Calcium Citrate: Sample - Not For Official UseDocument1 pageCalcium Citrate: Sample - Not For Official UseTanatorn TongsumrithNo ratings yet
- Determination of Hardness of Water and WastewaterDocument5 pagesDetermination of Hardness of Water and WastewaterThato NkhemeNo ratings yet
- Experiment 7 (EDTA) - Lab ManualDocument3 pagesExperiment 7 (EDTA) - Lab ManualJoseph JoeNo ratings yet
- Reviewer cm1231p PDFDocument5 pagesReviewer cm1231p PDFPark Shi Win0% (1)
- Enzyme-Technology-and-Biokinetics-Lab-Manual-BT-47L For Food LabDocument21 pagesEnzyme-Technology-and-Biokinetics-Lab-Manual-BT-47L For Food Labmasre semagnNo ratings yet
- Exp 2 chm421Document12 pagesExp 2 chm421Intan Sapura0% (1)
- Application of DC and Mark-Space Bias Differential Electrolytic Potentiometry For Determination of Cyanide Using A Programmable Syringe PumpDocument6 pagesApplication of DC and Mark-Space Bias Differential Electrolytic Potentiometry For Determination of Cyanide Using A Programmable Syringe PumpBhisma DamarekaNo ratings yet
- MB Assessment Act MBDocument2 pagesMB Assessment Act MBzedrickNo ratings yet
- Ion-Exchange Separation and Complex Metric Titration Determination of Nickel and CobaltDocument10 pagesIon-Exchange Separation and Complex Metric Titration Determination of Nickel and CobaltMuhammad AlghitanyNo ratings yet
- Procedures:: General Start-Up ProceduresDocument7 pagesProcedures:: General Start-Up ProceduresPaen ZulkifliNo ratings yet
- EPA Method 3101 PDFDocument3 pagesEPA Method 3101 PDFJalpit MardenNo ratings yet
- Determination of Chloride in Water 4500DDocument3 pagesDetermination of Chloride in Water 4500Dpious_chemNo ratings yet
- EPA Method 3101Document3 pagesEPA Method 3101skrim240No ratings yet
- Potentiometric Titration of A Mixture of CL in BeerDocument11 pagesPotentiometric Titration of A Mixture of CL in BeerMinichNo ratings yet
- Final Exam Reviewer BIOKMAN 1T AY2019-2020-1Document2 pagesFinal Exam Reviewer BIOKMAN 1T AY2019-2020-1Macy MarianNo ratings yet
- Sample Problems in Quality Control 1Document17 pagesSample Problems in Quality Control 1Jaica Mangurali TumulakNo ratings yet
- S003602441112003XDocument5 pagesS003602441112003Xvestuario17.1No ratings yet
- Calibration and MaintainanceDocument6 pagesCalibration and MaintainancePranyusha VeluriNo ratings yet
- CCC CC CCC CC CCC CCCDocument8 pagesCCC CC CCC CC CCC CCCfaznil100% (3)
- No 3Document12 pagesNo 3Punit Ratna ShakyaNo ratings yet
- Chemistry 251 Review Questions Exam I WordDocument2 pagesChemistry 251 Review Questions Exam I WordDavid Chambergo0% (2)
- Usp42-Nf37 2599Document1 pageUsp42-Nf37 2599RestiNo ratings yet
- Experiment 1 - Formal Report - Aguilar Alih BassarDocument15 pagesExperiment 1 - Formal Report - Aguilar Alih Bassarmedz dharNo ratings yet
- Chem 28.1 Midterm PSDocument2 pagesChem 28.1 Midterm PSAnonymous ee5dOjNo ratings yet
- Problem Set 4 SolutionDocument16 pagesProblem Set 4 SolutionJana PaduaNo ratings yet
- Analytical Chem Questions 2Document59 pagesAnalytical Chem Questions 2Ash Yehia50% (2)
- Mohr Method: Determination of ChlorideDocument2 pagesMohr Method: Determination of ChlorideHocPoLab TechNo ratings yet
- Experiment 2 - Analysis of An Unknown Vinegar SampleDocument8 pagesExperiment 2 - Analysis of An Unknown Vinegar SampleHanis Ridzuan100% (1)
- Ppotentiometric Titration of Benzoic Acid With 0.1M Sodium HydroxideDocument23 pagesPpotentiometric Titration of Benzoic Acid With 0.1M Sodium HydroxideCristine ConcepcionNo ratings yet
- AOAC967 - 30 - Ca y MGDocument1 pageAOAC967 - 30 - Ca y MGMaría De Los Ángeles SuárezNo ratings yet
- Arc Anachem QuestionsDocument4 pagesArc Anachem QuestionsJoshua Daniel SolomonNo ratings yet
- Lab ReportDocument5 pagesLab ReportMustapha AmineNo ratings yet
- Analytical Chemistry 14-6-20Document15 pagesAnalytical Chemistry 14-6-20Zakiah Dzul UmmahNo ratings yet
- Nucleic Acid ExperimentDocument25 pagesNucleic Acid ExperimentEinah Einah0% (1)
- Challenging Experiments Lab Manual-CurtDocument20 pagesChallenging Experiments Lab Manual-Curtpramukhjain.9966No ratings yet
- Purpose:: PH Testing and Alkalinity Determination Using The Titrametric MethodDocument4 pagesPurpose:: PH Testing and Alkalinity Determination Using The Titrametric MethodSamir PatelNo ratings yet
- Lab DiscussionDocument7 pagesLab DiscussionZeinab A. ElBhnsawiNo ratings yet
- Problems TitrationDocument2 pagesProblems TitrationThy AnhNo ratings yet
- Application of Ion Exchange ResinDocument3 pagesApplication of Ion Exchange ResinEdna Lip AnerNo ratings yet
- Experiment 4 2000Document8 pagesExperiment 4 2000قدس العجميNo ratings yet
- PH, EC and TDSDocument7 pagesPH, EC and TDSBrijesh Kumar MishraNo ratings yet
- SOP For Water TestingDocument50 pagesSOP For Water Testinggreen solutionNo ratings yet
- Tutorial Water CHEM136 OnlineDocument2 pagesTutorial Water CHEM136 OnlineVatsal AggarwalNo ratings yet
- Determination of The Alcohol LabDocument3 pagesDetermination of The Alcohol LabVIctoriakay100% (1)
- Adamson University College of EngineeringDocument14 pagesAdamson University College of EngineeringJosef RentaNo ratings yet
- 09.11.2010 MethodologyDocument16 pages09.11.2010 MethodologyavvaimsvijayaNo ratings yet
- ION EXCHANGE Analysis Testing Procedures GeneralDocument50 pagesION EXCHANGE Analysis Testing Procedures GeneralJinalNo ratings yet
- Chapter 5 PDFDocument11 pagesChapter 5 PDFJun Elbert JaboliNo ratings yet
- Sample Problems in Quality Control 1Document17 pagesSample Problems in Quality Control 1John TecsonNo ratings yet
- Prof o Exp3Document9 pagesProf o Exp3Mbali MdlaloseNo ratings yet
- MKBS221. Report Template .Prac 4.BACTERIOPHAGESDocument4 pagesMKBS221. Report Template .Prac 4.BACTERIOPHAGESMbali MdlaloseNo ratings yet
- Assignment 1Document5 pagesAssignment 1Mbali MdlaloseNo ratings yet
- Murendeni Exp3Document7 pagesMurendeni Exp3Mbali MdlaloseNo ratings yet
- mkbs317 Group AssignDocument8 pagesmkbs317 Group AssignMbali MdlaloseNo ratings yet
- Structure - Problem - 2 NCHE 311 2022 MEMODocument1 pageStructure - Problem - 2 NCHE 311 2022 MEMOMbali MdlaloseNo ratings yet
- Making An Agarose Gel.Document17 pagesMaking An Agarose Gel.Mbali MdlaloseNo ratings yet
- NCHE312 - MC Jun2022 1Document23 pagesNCHE312 - MC Jun2022 1Mbali MdlaloseNo ratings yet
- NCHE 211 UNIT 1 A 2022Document23 pagesNCHE 211 UNIT 1 A 2022Mbali MdlaloseNo ratings yet
- Practical 3 Introduction VideoDocument1 pagePractical 3 Introduction VideoMbali MdlaloseNo ratings yet
- Accuracy ExpirementDocument3 pagesAccuracy ExpirementMbali MdlaloseNo ratings yet