Professional Documents
Culture Documents
Sommerfeld - Conditions For Allowed Ellliptic Orbits
Uploaded by
zionahirene0 ratings0% found this document useful (0 votes)
1 views2 pagesSommerfeld atomic model
Original Title
Sommerfeld - Conditions for allowed ellliptic orbits
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentSommerfeld atomic model
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
1 views2 pagesSommerfeld - Conditions For Allowed Ellliptic Orbits
Uploaded by
zionahireneSommerfeld atomic model
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 2
Sommerfeld’s relativistic atom model:
To explain the observed fine structure of spectral lines, Sommerfeld introduced
two main modification in Bohr’s theory.
(1) According to Sommerfeld, the path of an electron around the nucleus, in
general, is elliptic i.e., an electron revolve round the nucleus in elliptic orbits with
the nucleus at one of the foci. The circular orbits are a special case.
(2) The velocity of electron moving in an elliptic orbit varies considerably at
different parts of the orbit. This causes relativistic variation in the mass of the
𝑚0
moving electron {v=2.2x106 ms-1, 𝑚 = 2
}. Therefore he took into account
√1−𝑣2
𝑐
the relativistic variation of the mass of the electron with velocity. Hence this
model is also called as relativistic atom model.
Theory:
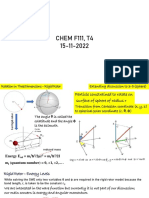
Consider the electron moving in an elliptic orbit around
the nucleus. Its position at any instant is described in
terms of polar coordinates r and φ as shown in figure.
Since r and φ both varies in an elliptical orbit, the
electron is endowed with two momentums with
changing r and φ i.e., radial momentum Pr and azimuthal
or orbital momentum Pφ. According to Bohr’s theory, these two momenta should
be quantised. The two quantisation condition are
∫ 𝑃𝑟 𝑑𝑟 = nr h ......... (1) and ∫ 𝑃𝜑 𝑑𝜑 = nφ h ......... (2)
Where nr is called the radial quantum number and nφ is called the angular or
azimuthal quantum number. nr and nφ take only positive integral values such that
nr + nφ = n, where n is the principal quantum number which takes the integral
values 1,2,3 ........∞.
Determination of allowed elliptical orbits:
The allowed elliptical orbits of an electron are determined by evaluating integrals
in equation (1) and (2).
Consider the integral in equation (2). The momentum 𝑃𝜑 corresponds to the
coordinate φ is merely the angular momentum P of the electron in elliptic orbit
which is constant, from Kepler’s law i.e., Pφ = P = constant. Integrating equation
(2) from 0 to 2π
2π 2π
∫0 𝑃𝜑 𝑑𝜑 = nφ h or 𝑃𝜑 ∫0 𝑑𝜑 = nφ h or
𝒏𝝋 𝒉
𝑷𝝋 = ........ (3)
𝟐𝝅
1 𝜖 𝑐𝑜𝑠𝜑
Consider the equation of ellipse in polar form = and substituting
𝑟 𝑎(1−𝜖 2 )
this in equation (1) and simplifying further we get the condition for allowed
elliptic orbits
𝑛∅2 𝑏2
1– ε =
2
2
, for an ellipse 1– ε2 = where a and b are semi-major and semi-
𝑛 𝑎2
minor axes respectively.
𝑏2 𝑛∅2 𝒃 𝒏∅
Hence
𝑎2
= 𝑛2 or = ........ (4)
𝒂 𝒏
Equation (4) is the condition that determines the allowed elliptical orbits. The
allowed elliptical orbits are those for which the ratio of semi-major and minor
axes is that of two integers. When
a) nφ = n implies b = a and the orbit becomes circular
b) nφ ≠ 0, since the ellipse would then degenerate into a straight line passing
through the nucleus.
c) nφ cannot be greater than n, since b is always than n.
Hence for a given value of n, nφ can take only n different values, which means
there can be only n elliptical orbits of different eccentricities.
For n=1, there is only one possible orbit which
is circular. For n=2, there are two possible
orbits, one circular and the other elliptic which
are designated as 21 and 22. For n=3, there are
3 possible orbits which are designated as 31, 32
and 33 and so on, the subscript being the
azimuthal quantum number nφ.
Fig. Permitted electron orbits
You might also like
- DocumentDocument6 pagesDocumentzahid- techNo ratings yet
- L3 Che101Document28 pagesL3 Che101Musa Ahammed MahinNo ratings yet
- Atomic Models Seminar by Shanti SharmaDocument24 pagesAtomic Models Seminar by Shanti Sharmaahsanbgayo100% (1)
- Rotational Motion: Rotation in Two Dimensions: A Particle On A RingDocument41 pagesRotational Motion: Rotation in Two Dimensions: A Particle On A RingAdelia Ayu WandiraNo ratings yet
- Polar Equations of Conics: Alternative Definition of ConicDocument7 pagesPolar Equations of Conics: Alternative Definition of ConicBeto LangNo ratings yet
- Lecture L16 - Central Force Motion: Orbits: Energy IntegralDocument8 pagesLecture L16 - Central Force Motion: Orbits: Energy IntegralKRITANTNo ratings yet
- Electronic Structure of The AtomDocument55 pagesElectronic Structure of The AtomAlekhoy Pakz100% (1)
- Lamb ShiftDocument28 pagesLamb ShiftMohammad zaid ZazNo ratings yet
- AtomsDocument12 pagesAtomsmidhunesh41No ratings yet
- Hydrogen-Deuterium Mass Ratio: 1 BackgroundDocument13 pagesHydrogen-Deuterium Mass Ratio: 1 Backgroundomkar makarNo ratings yet
- The Quantum Mechanical Model of An AtomDocument24 pagesThe Quantum Mechanical Model of An AtomKim Christian CombaterNo ratings yet
- Vasili Perebeinos and Philip B. Allen - Toward A Theory of Orbiton Dispersion in LaMnO3Document8 pagesVasili Perebeinos and Philip B. Allen - Toward A Theory of Orbiton Dispersion in LaMnO3SaqqerNo ratings yet
- Bohr's Atomic Model: I F I FDocument5 pagesBohr's Atomic Model: I F I FSantiago Lopez MurilloNo ratings yet
- Bangladesh Physics Olympiad 2016Document9 pagesBangladesh Physics Olympiad 2016Science Olympiad Blog88% (8)
- 8.321 Quantum Theory-I Fall 2016: Final Exam Dec 19, 2016Document4 pages8.321 Quantum Theory-I Fall 2016: Final Exam Dec 19, 2016bahadoor22i5583No ratings yet
- Asano 1975Document21 pagesAsano 1975Hamza AmjedNo ratings yet
- UCL Physics and Astronomy problem class atomic and molecular transitionsDocument4 pagesUCL Physics and Astronomy problem class atomic and molecular transitionsgodkid308No ratings yet
- Review of the isotope effect in the hydrogen spectrumDocument6 pagesReview of the isotope effect in the hydrogen spectrumNiraj KumarNo ratings yet
- Atomic Structure... (3) PPTDocument32 pagesAtomic Structure... (3) PPTVaibhav KargetiNo ratings yet
- The Two-Body Problem: Orbital MechanicsDocument23 pagesThe Two-Body Problem: Orbital MechanicsBellatrixMacabreNo ratings yet
- Atomic Structure 10feb07Document27 pagesAtomic Structure 10feb07Fredrick MutungaNo ratings yet
- 2023 L2 Waves and OrbitalsDocument67 pages2023 L2 Waves and OrbitalsVirginiaNo ratings yet
- Quick GuideDocument6 pagesQuick GuideAlonso BoteroNo ratings yet
- Indian Institute of Technology, Guwahati: CH101 Class 11 Physical ChemistryDocument5 pagesIndian Institute of Technology, Guwahati: CH101 Class 11 Physical ChemistryMihir Kumar MechNo ratings yet
- CH 6 (Cont'd)Document5 pagesCH 6 (Cont'd)PineraserNo ratings yet
- Feb 4 - 2Document10 pagesFeb 4 - 2MJ HidalgoNo ratings yet
- Atomic StructureDocument21 pagesAtomic StructureShahriar ShafiNo ratings yet
- Quantum Mechanics /quantum Numbers: Modern Concepts in Atomic StructureDocument9 pagesQuantum Mechanics /quantum Numbers: Modern Concepts in Atomic StructureManal.N.TNo ratings yet
- KeplerDocument7 pagesKeplerruloneggerNo ratings yet
- Chemistry Structure of AtomDocument81 pagesChemistry Structure of AtomlololberuhlololNo ratings yet
- Radial Wave Function and Angular Wave FunctionsDocument8 pagesRadial Wave Function and Angular Wave FunctionsBhavesh Garg100% (1)
- Section A_1 NotesDocument15 pagesSection A_1 NotesAniket HansNo ratings yet
- Quantum Numbers and Atomic OrbitalsDocument2 pagesQuantum Numbers and Atomic OrbitalsFrnyanaNo ratings yet
- Tight Binding ProjectDocument12 pagesTight Binding ProjectNitish KumarNo ratings yet
- Electron probability density and orbital shapesDocument30 pagesElectron probability density and orbital shapesbnkjayaNo ratings yet
- 5,6,7Document14 pages5,6,7عباسNo ratings yet
- 3 Assumptions and ApproximationsDocument5 pages3 Assumptions and ApproximationsJack RyderNo ratings yet
- Tarea 5Document2 pagesTarea 5Maria MontielNo ratings yet
- Inorganic Chem. I Ch. 1Document98 pagesInorganic Chem. I Ch. 1Shifa GhannamNo ratings yet
- Balmer Series Lab ReportDocument11 pagesBalmer Series Lab Reportsohaila gaberNo ratings yet
- Bohr's Model of the Hydrogen Atom ExplainedDocument5 pagesBohr's Model of the Hydrogen Atom Explainedevo84ukNo ratings yet
- Neutron Proton ScatteringDocument7 pagesNeutron Proton ScatteringDebayan DasguptaNo ratings yet
- CH 4-EM Waves in Anisotropic MediaDocument51 pagesCH 4-EM Waves in Anisotropic MediaFerrando NañezNo ratings yet
- Parametric Equations and Polar Coordinates 11.7. Conic Sections in Polar CoordinatesDocument5 pagesParametric Equations and Polar Coordinates 11.7. Conic Sections in Polar Coordinatessujit kcNo ratings yet
- Chapter 1 Electronic Strucutre - V2Document86 pagesChapter 1 Electronic Strucutre - V2MD.IRFAN UDDIN AHMED MEHEDINo ratings yet
- Different Atomic Models SeminarDocument24 pagesDifferent Atomic Models SeminarReddyvari VenugopalNo ratings yet
- Epicycloid Curve GeneratorDocument12 pagesEpicycloid Curve GeneratorJafar EarlNo ratings yet
- Quantum mechanical model explains atomic orbitalsDocument15 pagesQuantum mechanical model explains atomic orbitalsNatalia SadekNo ratings yet
- Cosmology in (2 + 1) -Dimensions, Cyclic Models, and Deformations of M2,1. (AM-121), Volume 121From EverandCosmology in (2 + 1) -Dimensions, Cyclic Models, and Deformations of M2,1. (AM-121), Volume 121No ratings yet
- Principles of Engineering Physics 1: Quantum Physics and Atom ModelsDocument12 pagesPrinciples of Engineering Physics 1: Quantum Physics and Atom ModelsDebanuj BasakNo ratings yet
- Shapes of OrbitalsDocument8 pagesShapes of Orbitalsopolla nianorNo ratings yet
- PHY-2251 (B) Physics - IV Chapter 8 on Atom ModelDocument18 pagesPHY-2251 (B) Physics - IV Chapter 8 on Atom Modeljoy bakshiNo ratings yet
- Wave Model - Hydrogen Case - 2023-2024Document36 pagesWave Model - Hydrogen Case - 2023-2024rahmaderradji23No ratings yet
- принстон 5 PDFDocument27 pagesпринстон 5 PDFLumpalump 300ftNo ratings yet
- Topic 5 QuantumDocument17 pagesTopic 5 Quantumizz isalahNo ratings yet
- II PUC Remedial Notes (2)Document32 pagesII PUC Remedial Notes (2)nithinganesh174No ratings yet
- BITS Pilani Chemistry Hamilton OperatorDocument11 pagesBITS Pilani Chemistry Hamilton OperatorNaresh SehdevNo ratings yet
- Schroedinger Equation Atomic Wave Functions Atomic Orbitals Quantum NumbersDocument45 pagesSchroedinger Equation Atomic Wave Functions Atomic Orbitals Quantum NumbersRishit JakhariaNo ratings yet
- Structure of AtomDocument112 pagesStructure of AtomAnanya SinghNo ratings yet
- The Hydrogen Balmer Series and Rydberg ConstantDocument14 pagesThe Hydrogen Balmer Series and Rydberg ConstantAshish MauryaNo ratings yet
- Sondhi RMP 69 315 1997Document19 pagesSondhi RMP 69 315 1997leNo ratings yet
- TermoooDocument11 pagesTermoooJay-r Capate TanNo ratings yet
- The Method of Volume Averaging BookDocument8 pagesThe Method of Volume Averaging BookGovinda ReddyNo ratings yet
- Assignment 1 - Group B: Compton EffectDocument4 pagesAssignment 1 - Group B: Compton Effectadrijeet8debNo ratings yet
- CH19 - Planar Kinetics of A Rigid Impulse and Momentum - DynamicDocument48 pagesCH19 - Planar Kinetics of A Rigid Impulse and Momentum - DynamicRs sniperNo ratings yet
- Ads Solitons With Conformal Scalar Hair: Yves Brihaye, Betti Hartmann and Sardor TojievDocument19 pagesAds Solitons With Conformal Scalar Hair: Yves Brihaye, Betti Hartmann and Sardor TojievR DaniNo ratings yet
- Maths Class Xii Chapter 03 Matrices Practice Paper 03Document4 pagesMaths Class Xii Chapter 03 Matrices Practice Paper 03kumar pNo ratings yet
- Ce 41-Quiz 1Document5 pagesCe 41-Quiz 1CONNIE INQUIGNo ratings yet
- Sample IB Questions ThermalDocument8 pagesSample IB Questions ThermalEthan KangNo ratings yet
- 2ND Year Math Guess PaperDocument6 pages2ND Year Math Guess PaperM ShabanNo ratings yet
- Vectors Worksheet AA HLDocument6 pagesVectors Worksheet AA HLfootball.hd.371No ratings yet
- Thermodynamics Fundamentales Morales RodriguezDocument553 pagesThermodynamics Fundamentales Morales RodriguezJorge Cronwell Montaño VásquezNo ratings yet
- Problem 3.18: SolutionDocument2 pagesProblem 3.18: SolutionEric KialNo ratings yet
- GR NotesDocument13 pagesGR NotesbbteenagerNo ratings yet
- Mid - Sem - 2019 - Linear Control SystemDocument1 pageMid - Sem - 2019 - Linear Control SystemGaurav SinghNo ratings yet
- Goldstein Classical Physics PDFDocument3 pagesGoldstein Classical Physics PDFRyanNo ratings yet
- Dual Superconductivity in Abelian Higgs Model of QCD: B. S. RajputDocument16 pagesDual Superconductivity in Abelian Higgs Model of QCD: B. S. Rajputmohamed salahNo ratings yet
- Thermal Physics: Golam Dastegir Al-QuaderiDocument37 pagesThermal Physics: Golam Dastegir Al-QuaderiTanvir KhanNo ratings yet
- Maths Revision DPP No 4Document8 pagesMaths Revision DPP No 4Praphul Pulkit GiriNo ratings yet
- BEP Shayan Farrokhi Final VersionDocument93 pagesBEP Shayan Farrokhi Final VersionShahad Hussain Kavassery SakeerNo ratings yet
- 3 - Maths Bridge Course Material - 23-24 (16 04 23)Document33 pages3 - Maths Bridge Course Material - 23-24 (16 04 23)Sasi SasiNo ratings yet
- Orifice PlateDocument7 pagesOrifice PlatedombipinNo ratings yet
- The Equation of State For A Perfect Gas: A. Boyle'S LawDocument9 pagesThe Equation of State For A Perfect Gas: A. Boyle'S LawCzarina Jane PeregrinNo ratings yet
- 5 Temp Ideal Gas-Fall 2022Document22 pages5 Temp Ideal Gas-Fall 2022asakr8481No ratings yet
- Albert Einstein Fact SheetDocument1 pageAlbert Einstein Fact SheetCeren ManasNo ratings yet
- Thermodynamics Solutions Chapter 12Document44 pagesThermodynamics Solutions Chapter 12RG RAJNo ratings yet
- Lecture 2 - Heat and Mass Transfer (HMT) - 5th Sem - ME1053Document28 pagesLecture 2 - Heat and Mass Transfer (HMT) - 5th Sem - ME1053Monkey D. LuffyNo ratings yet
- Assignment 2 PDFDocument1 pageAssignment 2 PDFSai Prasanna100% (1)
- Chap 5Document5 pagesChap 5Luis David Cajavilca CutimancoNo ratings yet
- Kinetic Theory of an Ideal Gas ExplainedDocument45 pagesKinetic Theory of an Ideal Gas ExplainedTâm NguyễnNo ratings yet