Professional Documents
Culture Documents
Chapter 3 Periodic Table (SUEC) Answer
Uploaded by
Lee AustinOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Chapter 3 Periodic Table (SUEC) Answer
Uploaded by
Lee AustinCopyright:
Available Formats
Chapter 3: Periodic Table (SUEC)
3.1 Electronic configuration and the Periodic Table
- The elements in the Periodic Table can be divided into four blocks depending on their
valence shell electronic configuration.
- They are the s-block, p-block, d-block and f-block elements.
- All elements in the same group have the same number of valence electrons with similar
electronic configuration.
The s-block elements
- Consists of elements from Group 1 and Group 2.
- Group 1 elements (alkaline metal) have one valence electron in the outermost orbital
with the configuration of s1.
- Group 2 elements (alkaline earth metal) have two electrons in the outermost orbital
with the configuration of s2.
The p-block elements
- Elements of Group 13 to Group 18 are known as the p-block elements because the
outermost orbitals that are filled with electrons are the p orbitals.
- They have valence shell electronic configuration of s2p1 to s2p6
The d-block elements (transition element)
- Elements of Group 3 to Group 12 are known as d-block elements.
- They have valence shell electronic configuration of d1s2 to d10s2
- They are typical metals with very high melting points and boiling points.
Sr1 Chemistry SUEC 2020 1 Chap 3: Periodic Table
The f-block elements
- Elements with proton number 58-71 (known as lanthanides) and from 90-103 (known
as actinides) are f-block elements.
- They have valence shell electronic configuration of f1d10s2 to f14d10s2
Valence shell configuration from the periodic table
- Most chemical reactions involve only the valence shell electrons. Hence, the valence
shell configuration of an element is important in predicting the chemical properties of
an element.
- The group number of an element indicates the number of valence electrons while
the period number indicates the outermost principle shell that if filled with
electrons.
- For example: aluminium (Z = 13) is in Group 13 (IIIA) and Period 3.
➢ This means the outermost energy level that is filled with electrons is the 3rd shell,
and it has 3 valence electrons (not 13).
➢ Hence, its valence shell configuration is 3s23p1
Sr1 Chemistry SUEC 2020 2 Chap 3: Periodic Table
Test yourself 1
Write the valence shell electronic configuration of the following elements.
Valence shell electronic
Element Group number Period number
configuration
X 17 4 4s24p5
Y 1 6 6s1
Z 18 2 2s22p6
3.2 Atomic radius and ionic radius
- The atomic radius is half the distance the nuclei of two atoms of a metallic element.
- For molecular elements, the atomic radius is half the distance between the nuclei of
the two atoms joined by covalent bond.
- Atomic radius depends on two major factors:
(a) The nuclear charge
➢ The higher the nuclear charge, the stronger the attraction between the nucleus and
the electron cloud, cause the atomic size to decrease.
(b) The screening effect
➢ Depends on number of shells that are filled with electrons.
➢ Inner electrons can effectively screen the outer electrons from the pull of nucleus
➢ Increasing screening effect causes the size of the atoms to increase.
Sr1 Chemistry SUEC 2020 3 Chap 3: Periodic Table
Variation of atomic radius across a period
- For atoms in the same period, the number of protons and electrons increase by one each.
However, each additional electron is added to the same shell, hence, the screening
effect does not change much.
- However, the increasing nuclear charge will exert a stronger attraction between the
nucleus and electron cloud, causing the atomic size to decrease.
- Atoms of Period 3 elements are bigger than the corresponding elements in Period 2
because they have one extra inner shell that is filled with electrons, increasing the
screening effect.
Variation of atomic radius going down a group
- Going down the group, both the nuclear
charge and the screening effect
increase.
- However, the increase in the screening
effect is larger than the increase in
nuclear charge, leads to a decrease in
the effective nuclear charge.
- The attraction between the nucleus and
the electron cloud gets weaker, causing
atomic size to increase.
Sr1 Chemistry SUEC 2020 4 Chap 3: Periodic Table
Variation of ionic radius across Period 3
- The ionic radius is the radius of a cation or an anion.
- The anions are all larger than the cations because the anions have one extra shell filled
with electrons compared to the cations.
Ion Na+ Mg2+ Al3+ P3- S2- Cl-
Ionic radius(nm) 0.095 0.065 0.050 0.212 0.184 0.181
- The cation size decreases with increasing proton number.
- Because all the cations have 10 electrons but the number of protons increases from 11 to
13. Hence attraction between the nucleus and the electron increases.
- The same applies to the anions.
Variation of ionic radius down a group
- Going down group, each successive ion
has one extra shell filled with electrons.
- The increase in screening effect causes
the ionic size to increase.
Sr1 Chemistry SUEC 2020 5 Chap 3: Periodic Table
Test yourself 2
1. Arrange the following species in order of increasing size
(a) N, N3- ______________ N< N3- ____________
(b) Fe, Fe2+, Fe3+ _________ Fe3+ < Fe2+ < Fe ______________________
2. Predict which ion in the following pairs has a bigger size.
(a) N3- and F- _______________________________
(b) Mg2+ and K+ _______________________________
Answer:
Sr1 Chemistry SUEC 2020 6 Chap 3: Periodic Table
3.3 Electronegativity
- Electronegativity of an element is a measure of the ability of the atom to attract the
electrons in a covalent bond to which it is bonded.
- Electronegativity
(a) increases across a period
(b) decrease down a group
- Going across a period from left to right, the atomic size decreases, while the nuclear charge
increases.
➢ the attraction for electrons become stronger and the electronegativity increases.
- Going down a group, the atomic size increases while the effective nuclear charge decreases.
➢ the attraction for electrons become weaker and the electronegativity decreases.
Sr1 Chemistry SUEC 2020 7 Chap 3: Periodic Table
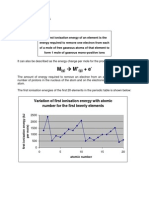
3.3 Ionisation energy
- The first ionisation energy of an element is the minimum energy required to remove one
electron from the outermost orbital of every atom in 1 mol of free gaseous atoms to form
1 mol of unipositive gaseous ions, under standard conditions.
M(g) → M+ (g) + e ∆H = first ionisation energy
M+ (g) → M2+ (g) + e ∆H = second ionisation energy
Factors affecting the ionisation energy
- The ease in removing an electron from an atom or ion depends on the attraction between
the nucleus and the electron
- Ionisation energy depends on the factors:
(a) The distance between the nucleus and the electron (i.e. atomic size)
➢ The attraction between the nucleus and electrons decreases with increasing distance
between them.
➢ Hence, the larger the size of an atom or ion, the lower the ionisation energy.
(b) The nuclear charge (i.e number of protons)
➢ The higher the nuclear charge, the stronger is the attraction between the nucleus and
the electrons. This causes the ionisation energy to increase.
(c) The screening effect
➢ When the number of inner shells that are filled with electrons increases, the valence
electrons are more shielded from the attraction of the nucleus.
➢ The repulsion between the inner electron shells will cause the atomic size to increase.
Hence, increasing screening effect will decrease the ionisation energy.
Trend of ionisation energy across a period
Sr1 Chemistry SUEC 2020 8 Chap 3: Periodic Table
- There is a general increase in the first ionisation energy with increasing proton
numbers for elements of Period 2 and 3.
- Going across a period from left to right, the atomic size decreases, the nuclear charge
increases, but the screening effect remains almost the same. Therefore, the electrons are
more tightly bound to nucleus and are more difficult to be removed.
- However, the increase is not uniform. The first ionisation energy of Be (period 2) and
Mg (Period 3) is higher than expected. This is because the first electron to be removed
from these atoms are from a fully filled s orbital:
Be: 1s2 2s2
Mg: 1s2 2s2 2p6 3s2
- The s2 configuration has greater stability thus making electron more difficult to be
removed than expected.
- The first ionisation energy of nitrogen (Period 2) and phosphorus (Period 3) is higher
than expected because the first electron removed from a completely half-filled p
orbital which also has greater stability.
N: 1s22s22p3
P: 1s22s22p63s23p3
- The first ionisation energy of Period 2 elements is higher than their corresponding
Period 3 elements because Period 2 elements are smaller in size than Period 3 elements.
Trend of ionisation energy down a group
- Going down a group, atomic size
increases while the effective nuclear
charge decreases. The attraction between
the nucleus and the electrons become
weaker.
- Hence, the first ionisation energy
decreases down the group.
Sr1 Chemistry SUEC 2020 9 Chap 3: Periodic Table
Chapter 3: Exercise (SUEC)
1. An element has the electronic configuration 1s22s22p63s23p63d104s24p64d105s25p5
a) Which block in the Periodic Table down this element belongs to? ____________________
b) Which group does it belong to? ______________________________
c) Identify this element. ________________________________
2. Element X has the electronic configuration 1s22s22p63s23p63d84s2
a) Which block in the Periodic Table does element X belong to? ____________________
b) State the maximum number of electrons in a d subshell. _________________________
c) Element X forms an ion with charge +2
d) Write the full electronic configuration for this ion.
_______________________________________________________________________
3. The table below gives some data for the Period 3 elements.
(a) Define the term first ionisation energy.
The energy required to remove one mole of electrons from one mole of atoms in
the gas state to make one mole of ions in gas state, each with single positive charge.
(b) Describe and explain the general trend of in first ionisation energy from left to right of
Period 3.
From left to right, the first ionisation energy increases. The increasing nuclear
charge and decreasing atomic radius result in stronger force of attraction between
the nucleus and the outer electrons.
(c) Give the electronic configurations of phosphorus and sulphur.
Sr1 Chemistry SUEC 2020 10 Chap 3: Periodic Table
(d) Describe and explain the trend in atomic radius from left to right of Period 3.
The atomic radius decreases from left to right. This is because the positive nuclear
charge increases from left to right and the increasing nuclear charge attracts
electrons in outer shell more strongly.
(e) Describe and explain the difference in atomic and ionic radius for aluminium.
The ionic radius is smaller than the atomic radius. The difference is due to the ion
has a greater number of positively charged protons than negatively charged
electrons, so electrons are attracted more strongly and pulled in more closely to
the nucleus.
Multiple choice questions
1. Which of the following graphs of first ionisation energy against proton number for the
Period 3 elements is correct? (B)
2. Which of the following graphs best represents the variation in the ionic radius of the
Periodic Table elements (from Na to Cl)? (A)
3. Which of the following ions has the largest radius?
A. Na+ B. Al3+ C. S2- D. Cl-
Sr1 Chemistry SUEC 2020 11 Chap 3: Periodic Table
4. The size of Na+, Mg2+, and Al3+ is in the order: Na+ > Mg2+ > Al3+. Which of the
following best explains this trend?
A. The number of electrons decreases while the number of protons increases.
B. The number of electrons are the same but the number of protons increases.
C. The number of electrons and protons decreases.
D. The number of electrons and protons increases.
5. Going across the Period 3 from sodium to chlorine,
A. The atomic radius increases
B. The electrical conductivity increases
C. The electronegativity increases
D. The melting point increases
Sr1 Chemistry SUEC 2020 12 Chap 3: Periodic Table
You might also like
- Periodic Table and PeriodicityDocument11 pagesPeriodic Table and Periodicityakeemoluwadamilare623No ratings yet
- Inorganic Chemistry NotesDocument105 pagesInorganic Chemistry NotesOdongo TonnyNo ratings yet
- A - Level Inorganic Chemistry 2-1Document171 pagesA - Level Inorganic Chemistry 2-1kitderoger_391648570No ratings yet
- Chem 111 Week Three Notes BDocument7 pagesChem 111 Week Three Notes BnyachiosirNo ratings yet
- A-LEVEL INORGANIC CHEMISTRY NOTESDocument55 pagesA-LEVEL INORGANIC CHEMISTRY NOTESaudrey abaasaNo ratings yet
- PERIODIC TRENDS OF ATOMIC PROPERTIESDocument31 pagesPERIODIC TRENDS OF ATOMIC PROPERTIESKeshan PaudelNo ratings yet
- Chapter 2 - Electrons in AtomsDocument32 pagesChapter 2 - Electrons in AtomsFandyNo ratings yet
- A039level Chemistry Inorganic NotesDocument108 pagesA039level Chemistry Inorganic NotesNasser SsennogaNo ratings yet
- A-Level Chemistry NotesDocument10 pagesA-Level Chemistry NotesHannah Bryson-JonesNo ratings yet
- Unit Objectives: Periodic TrendsDocument9 pagesUnit Objectives: Periodic Trendsmartin mulengaNo ratings yet
- F321 PeriodicityDocument3 pagesF321 PeriodicityDoc_CrocNo ratings yet
- Inorganic Chemistry STPMDocument113 pagesInorganic Chemistry STPMThilagavathy SethuramahNo ratings yet
- INORGANIC Periodic Table 1Document26 pagesINORGANIC Periodic Table 1ThilagaNo ratings yet
- Inorganic Chemistry NotesDocument73 pagesInorganic Chemistry NotesYT ChongNo ratings yet
- Classification of ElementsDocument74 pagesClassification of ElementsJimit Patel BankNo ratings yet
- Engineering Chemistry Notes UNIT 4Document9 pagesEngineering Chemistry Notes UNIT 4Nivetha ENo ratings yet
- CIE Chemistry A Level: 2: Atomic StructureDocument6 pagesCIE Chemistry A Level: 2: Atomic StructureMyraNo ratings yet
- CIE Chemistry A Level: 2: Atomic StructureDocument6 pagesCIE Chemistry A Level: 2: Atomic StructureahumanbeinginearthNo ratings yet
- LEARNING ACTIVITY SHEET-CHEM 1 q1 Week 7Document20 pagesLEARNING ACTIVITY SHEET-CHEM 1 q1 Week 7Jhude JosephNo ratings yet
- Chem Inorganic A'levelDocument95 pagesChem Inorganic A'levelAlvin HavinzNo ratings yet
- A Level Inorganic Chemistry NotesDocument95 pagesA Level Inorganic Chemistry NotesnaluwairoericjohnNo ratings yet
- Chap 1.1Document48 pagesChap 1.1Irfan AzaharNo ratings yet
- Chemistry Form 6 Sem 2 03Document45 pagesChemistry Form 6 Sem 2 03Ng Swee Loong StevenNo ratings yet
- What is a Mass SpectrometerDocument11 pagesWhat is a Mass SpectrometerFiliz Kocayazgan ShanablehNo ratings yet
- Chapter 7 Jan13Document98 pagesChapter 7 Jan13kumuthaNo ratings yet
- Periodic Table PropertiesDocument45 pagesPeriodic Table PropertiesUMMU MARDHIAH ABDUL HALIMNo ratings yet
- Chapter 03 PeriodicityDocument116 pagesChapter 03 PeriodicityJishen ZhuNo ratings yet
- Patterns of First Ionisation Energies in The Periodic TableDocument7 pagesPatterns of First Ionisation Energies in The Periodic TableRugen RajNo ratings yet
- Chemical Classification and Periodic TrendsDocument20 pagesChemical Classification and Periodic TrendsRAJEEV KARAKOTINo ratings yet
- Atomic Structure and Periodic Table Key ConceptsDocument4 pagesAtomic Structure and Periodic Table Key Conceptsjulian maltoNo ratings yet
- AQA Chemistry A-Level: 3.2.1: PeriodicityDocument4 pagesAQA Chemistry A-Level: 3.2.1: PeriodicityTakundanashe MpamboNo ratings yet
- TOPIC 2. Atomic Structure and Periodic TableDocument11 pagesTOPIC 2. Atomic Structure and Periodic TableVictor VictorNo ratings yet
- Che Chapter 3Document7 pagesChe Chapter 3lisaNo ratings yet
- First Ionisation Energies (FIEsDocument6 pagesFirst Ionisation Energies (FIEsAathifa ThowfeekNo ratings yet
- Ion EnergiesDocument4 pagesIon EnergiesNamratha NairNo ratings yet
- Periodicity Trends Across Periods 2 and 3Document7 pagesPeriodicity Trends Across Periods 2 and 3Anshu MovvaNo ratings yet
- Btech Chem NIFFT Part 1 PDFDocument53 pagesBtech Chem NIFFT Part 1 PDFকৃ ষ্ণাNo ratings yet
- Unit1 PP CFT Mot Notes PDFDocument18 pagesUnit1 PP CFT Mot Notes PDFThota KeerthiNo ratings yet
- Test #3 AnswersheetDocument4 pagesTest #3 AnswersheetRaymond TambiliNo ratings yet
- Review of Atomic Structure: Chapter Outline Nature of Interatomic BondingDocument8 pagesReview of Atomic Structure: Chapter Outline Nature of Interatomic BondingCharmis EnriquezNo ratings yet
- Atomic Structure and Bonding FundamentalsDocument27 pagesAtomic Structure and Bonding FundamentalsAdrian G. ClaritoNo ratings yet
- Self HelpDocument33 pagesSelf HelpHimanshu KumarNo ratings yet
- An Overview of The Properties of Elements in The Periodic Table The Key Atomic PropertiesDocument8 pagesAn Overview of The Properties of Elements in The Periodic Table The Key Atomic PropertiesMichelle MalabananNo ratings yet
- Pioneer Junior College Higher 2 Chemistry (9647) Inorganic Chemistry The Periodic Table: Chemical PeriodicityDocument22 pagesPioneer Junior College Higher 2 Chemistry (9647) Inorganic Chemistry The Periodic Table: Chemical PeriodicityTimothy HandokoNo ratings yet
- UntitledDocument27 pagesUntitledFatimah AgboolaNo ratings yet
- Chapter 3 NotesDocument7 pagesChapter 3 NotesNickNo ratings yet
- Periodic Trends in Physical and Chemical PropertiesDocument68 pagesPeriodic Trends in Physical and Chemical PropertiesAnisha Syazwana Binti RoslyNo ratings yet
- 3.2 Periodicity (STUDENT) Edited 20apr2017 PDFDocument116 pages3.2 Periodicity (STUDENT) Edited 20apr2017 PDFAliffuddin MohamadNo ratings yet
- 3.2 Periodicity PDFDocument86 pages3.2 Periodicity PDFnurulnadzirah_99100% (1)
- The Electron Density in An Atom Extends Far Beyond The Nucleus But We Define The Size of An Atom in Terms of Its Atomic Radius - One-Half TheDocument31 pagesThe Electron Density in An Atom Extends Far Beyond The Nucleus But We Define The Size of An Atom in Terms of Its Atomic Radius - One-Half TheAhRun5171No ratings yet
- Periodic Trends C12 2 07Document13 pagesPeriodic Trends C12 2 07Kuro NekoNo ratings yet
- Periodicity of Physical PropertiesDocument1 pagePeriodicity of Physical PropertiesGreen SlimeNo ratings yet
- Nuclear Energy ModuleDocument24 pagesNuclear Energy ModuleJessalyn PaclebNo ratings yet
- As Chemistry 宝典 2023新版 Daisy-黑白5本 1Document80 pagesAs Chemistry 宝典 2023新版 Daisy-黑白5本 1bbcdgg1234567No ratings yet
- Nuclear Charge Increases.: (Do Not Mention Shielding Effect)Document3 pagesNuclear Charge Increases.: (Do Not Mention Shielding Effect)sfndmnfmnNo ratings yet
- Periodic Properties Copy 2Document30 pagesPeriodic Properties Copy 2Rodayna HosniNo ratings yet
- HL Electron Configuration and The Periodic TableDocument12 pagesHL Electron Configuration and The Periodic TableMarilee HuntNo ratings yet
- Periodic Properties: Chemistry For (I.I.T. J.E.E.)Document11 pagesPeriodic Properties: Chemistry For (I.I.T. J.E.E.)Gagan Raj JaiswalNo ratings yet
- Chemistry 103 Assignment No. 6: As at As AsDocument3 pagesChemistry 103 Assignment No. 6: As at As AsAli EslamiNo ratings yet
- CPLRDocument2 pagesCPLRsofyanshahNo ratings yet
- Test Bank The Human Body Health Illness 5th Edition HerlihyDocument15 pagesTest Bank The Human Body Health Illness 5th Edition HerlihyRhonda Mosholder100% (22)
- Radiation Chemistry: 22.55 "Principles of Radiation Interactions"Document17 pagesRadiation Chemistry: 22.55 "Principles of Radiation Interactions"Muhammad SohilNo ratings yet
- General Chemistry 2: The Structure of Crystalline and Amorphous SolidsDocument55 pagesGeneral Chemistry 2: The Structure of Crystalline and Amorphous Solidsmary joy nemenzoNo ratings yet
- Chloride 2 PDFDocument9 pagesChloride 2 PDFJustin JonanyNo ratings yet
- Syntheses Vanillyl AlcoholsDocument48 pagesSyntheses Vanillyl AlcoholsIlmuncMakesuill100% (1)
- Rosin Based Chemicals and PolymersDocument44 pagesRosin Based Chemicals and PolymersHimanshu PanchalNo ratings yet
- Valency IonsDocument14 pagesValency IonsMojdeh AnbarfamNo ratings yet
- hw06 SolutionsDocument6 pageshw06 SolutionsChristina Ish SpeshNo ratings yet
- Zeolites in Water Treatment PDFDocument32 pagesZeolites in Water Treatment PDFsili11No ratings yet
- Oxidation Numbers, Redox and Half Equations PDFDocument6 pagesOxidation Numbers, Redox and Half Equations PDFRabia RafiqueNo ratings yet
- Solid State Physics MCQsDocument7 pagesSolid State Physics MCQsAhsan MoinNo ratings yet
- Periodic Table WorksheetDocument4 pagesPeriodic Table Worksheettony zouNo ratings yet
- Investigations On The Theory of The Photographic Process - Sheppard and MeesDocument366 pagesInvestigations On The Theory of The Photographic Process - Sheppard and MeesJorgeCamachoNo ratings yet
- Chemical BondsDocument28 pagesChemical Bondsmichaeldevid7890No ratings yet
- Relative Plugging IndexDocument10 pagesRelative Plugging Indexspurwito46No ratings yet
- Chemical Dictionary PDFDocument79 pagesChemical Dictionary PDFafb40% (1)
- Electron configurations and ionic bonding overviewDocument3 pagesElectron configurations and ionic bonding overviewFrancesca Irah MapaNo ratings yet
- Ret-7 (Champ) - (1ST Year) - 2017 (P2) - 19.08.2019Document16 pagesRet-7 (Champ) - (1ST Year) - 2017 (P2) - 19.08.2019rohan jhaNo ratings yet
- AP Chemistry - 03 NMSI Chemical Nomenclature STUDENTDocument14 pagesAP Chemistry - 03 NMSI Chemical Nomenclature STUDENTAbdul SamiNo ratings yet
- Module 3 - Electrical Fundamentals - CAT - B1 - B2Document192 pagesModule 3 - Electrical Fundamentals - CAT - B1 - B2aakashbarot100% (2)
- Grade 9 Q2 ReviewerDocument30 pagesGrade 9 Q2 Reviewerchrxtine hernandoNo ratings yet
- CONDUCTOMETRYDocument10 pagesCONDUCTOMETRYfatima maqboolNo ratings yet
- Solid Polymer Electrolytes: Materials Designing and All-Solid-State Battery Applications: An OverviewDocument20 pagesSolid Polymer Electrolytes: Materials Designing and All-Solid-State Battery Applications: An OverviewA1234 AJEFNo ratings yet
- Periodic Classification of Elements & Periodicity ExplainedDocument148 pagesPeriodic Classification of Elements & Periodicity ExplainedMuhammad Ismail100% (1)
- Faradays 1 Law of Electrolysis States That The Mass of A Substance Produce, Liberated At/ or DissolvedDocument3 pagesFaradays 1 Law of Electrolysis States That The Mass of A Substance Produce, Liberated At/ or DissolvedAlex noslenNo ratings yet
- Mechanism of electrophilic aromatic substitution reaction studied by ab initio calculationsDocument17 pagesMechanism of electrophilic aromatic substitution reaction studied by ab initio calculationsIvanAgustínAymerichNo ratings yet
- PERIODIC TABLE TESTDocument2 pagesPERIODIC TABLE TESTLama DebanaNo ratings yet