Professional Documents
Culture Documents
Nerves Distribution
Uploaded by
deargrace2512Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Nerves Distribution
Uploaded by
deargrace2512Copyright:
Available Formats
Blackwell Publishing IncMalden, USAJSMJournal of Sexual Medicine1743-6095© 2006 International Society for Sexual MedicineSeptember/October 200636979987Original ArticleNerve Distribution
in the VaginaPauls et al.
979
ORIGINAL RESEARCH—BASIC SCIENCE
A Prospective Study Examining the Anatomic Distribution of Nerve
Density in the Human Vagina
Rachel Pauls, MD,* George Mutema, MD,† Jeffrey Segal, MD,‡ W. Andre Silva, MD,§
Steven Kleeman, MD,* Vicki Dryfhout, MA,¶ and Mickey Karram, MD*
*Division of Urogynecology and Reconstructive Pelvic Surgery, Good Samaritan Hospital, Cincinnati, OH, †Department of
Pathology, Good Samaritan Hospital, Cincinnati, OH, ‡Saint Barnabas Medical Center—Center for Urogynecology,
Livingston, NJ, §Pacific Northwest Urogynecology—Urogynecology, Federal Way, WA, ¶Hatton Institute for Research and
Education, Good Samaritan Hospital, Cincinnati, OH, USA
DOI: 10.1111/j.1743-6109.2006.00325.x
ABSTRACT
Introduction. Women possess sufficient vaginal innervation such that tactile stimulation of the vagina can lead to
orgasm. However, there are few anatomic studies that have characterized the distribution of nerves throughout the
human vagina.
Aim. The aim of this prospective study was to better characterize the anatomic distribution of nerves in the adult
human vagina. A secondary aim was to assess whether vaginal innervation correlates with the subject’s demographic
information and sexual function.
Methods. Full-thickness biopsies of anterior and posterior vagina (proximal and distal), cuff, and cervix were taken
during surgery in a standardized manner. Specimens were prepared with hematoxylin and eosin, and S100 protein
immunoperoxidase. The total number of nerves in each specimen was quantified. Enrolled patients completed a
validated sexual function questionnaire (Female Sexual Function Index, FSFI) preoperatively.
Main Outcome Measures. A description of vaginal innervation by location and an assessment of vaginal innervation
in association with the subject’s demographic information and sexual function.
Results. Twenty-one patients completed this study, yielding 110 biopsy specimens. Vaginal innervation was some-
what regular, with no site consistently demonstrating the highest nerve density. Nerves were located throughout
the vagina, including apex and cervix. No significant differences were noted in vaginal innervation based on various
demographic factors, including age, vaginal maturation index, stage of prolapse, number of vaginal deliveries, or
previous hysterectomy. There were no correlations between vaginal nerve quantity and FSFI domain and overall
scores. Fifty-seven percent of the subjects had female sexual dysfunction; when compared to those without dysfunc-
tion, there were no significant differences in total or site-specific nerves.
Conclusions. In a prospective study, vaginal nerves were located regularly throughout the anterior and posterior
vagina, proximally and distally, including apex and cervix. There was no vaginal location with increased nerve density.
Vaginal innervation was not associated with demographic information or sexual function. Pauls R, Mutema G,
Segal J, Silva WA, Kleeman S, Dryfhout V, and Karram M. A prospective study examining the anatomic
distribution of nerve density in the human vagina. J Sex Med 2006;3:979–987.
Key Words. Anatomy (Gross and Microscopic); Central Nervous System Control; Neurophysiologic Studies of
Sexual Function
Introduction canalization, and resorption. In the XX fetus,
absence of müllerian-inhibiting factor and test-
he human vagina develops in a stepwise fash- osterone leads to elongation of the uterovaginal
T ion via the processes of elongation, fusion, primordium. This primordium meets the distal
© 2006 International Society for Sexual Medicine J Sex Med 2006;3:979–987
980 Pauls et al.
urogenital sinus to form the vaginal plate. Central The purpose of vaginal innervation is unclear.
erosion occurs to create a vaginal lumen, which is These nerves may be necessary for cervical dila-
complete by the 20th week of gestation [1]. tion and subsequent vaginal delivery; altered
Although controversial, most studies report that cervical innervation in late pregnancy has been
the müllerian ducts form the upper third of the reported [13]. After childbearing is complete, the
vagina whereas the lower two-thirds derive from vagina functions as an organ for sexual intimacy
the urogenital sinus [2,3]. and intercourse. Vaginal eroticism has been docu-
The neurophysiology of the vagina is poorly mented, with both the anterior and posterior
understood. Studies using animal models have vagina noted to elicit orgasmic responses [14–16].
demonstrated that fibers from the pudendal, pel- Some have commented on a “vaginal pacemaker”
vic, and hypogastric nerves innervate the pelvic that exists at the upper vagina, is responsible for
organs [4]. The pudendal nerve provides innerva- vaginal contractile activity, and may represent an
tion for the perineum, clitoris, and urethra, and area of erotic sensitivity [17]. Intact neural path-
pelvic nerve sensory fibers innervate the vagina. ways are essential for sexual responses, such as
These fibers have the greatest concentration in arousal and lubrication, and subjects with neuro-
the vaginal fornix [5]. In the rat vagina, large num- logical disorders often have problems with sexual
bers of nerves demonstrating branching toward function [18,19]. Based on these reports, it is pos-
the epithelium have been documented distally, sible that vaginal sensation and sexual responses
whereas no such fibers were observed in the prox- are mediated by nerve fibers terminating in the
imal vagina. Rich innervation of various struc- vaginal mucosa; however, this has not been
tures, especially vaginal arteries, has been noted described previously. Indeed, although a relation-
[6]. Estrogen levels may play a role in this inner- ship between pudendal nerve integrity and sexual
vation. Some studies have shown that increased function has been suggested [20], there is no sci-
estrogen leads to expansion of the size and sensi- entific evidence that vaginal innervation is corre-
tivity of pudendal perineal nerves [4], and causes lated with symptoms of sexual function.
alterations in rat vaginal mucosa morphology and The aim of this study was to provide a descrip-
contractility [7]. However, others reported no tion of nerve distribution and density throughout
change in nerve density based on estradiol admin- the vagina. A secondary aim was to determine
istration, ovariectomy, or estrous cycle in rat vag- whether there was an association between vaginal
inal tissue [6,8]. innervation and the subject’s demographic infor-
Few studies are available using women. Initial mation, such as age, vaginal estrogenization, pre-
work by Krantz suggested that “ganglion cells” vious hysterectomy, and sexual function.
were in the adventitia surrounding the vagina and
along the lateral walls next to the blood supply [9].
Material and Methods
They were seen in the upper third of the vagina
below the bladder. However, only sparse free This was a prospective study of patients undergo-
nerve endings associated with pain were seen in ing vaginal surgery for prolapse and incontinence
the epithelium and muscularis [9]. In the 1960s, between February and August 2004. Eligibility
Owman and colleagues [10] evaluated the vaginal criteria included sexual activity (defined as sexual
cuff of adult women undergoing hysterectomy. intercourse in a stable, heterosexual relationship)
They described few adrenergic nerve terminals in in the prior 4 weeks and planned surgery involving
these cuff specimens [10]. Later work utilizes at minimum an anterior and posterior colporrha-
immunohistochemical stains to improve visualiza- phy. Subjects were excluded if they had diabetes
tion of vaginal nerves. Hilliges et al. [11] evaluated or any neurological condition, or if surgery was
six sites in the vaginal mucosa using protein gene converted to an abdominal approach. During
product 9.5 staining. They reported increased these months, nearly 60 patients were scheduled
innervation in the distal and anterior vaginal wall for this surgery, and approximately half were sex-
[11]. A more recent study outlined an analysis of ually active. All eligible patients were advised of
female fetal specimens. They demonstrated that the study, and all agreed by written informed con-
branches of the inferior hypogastric plexus extend sent. A Pelvic Organ Prolapse––Quantification
on the lateral walls of the vagina, are most dense (POP-Q) examination was performed in the stan-
on the mid and proximal vagina, and travel supe- dard fashion [21], and preoperative urodynamic
riorly to cover the proximal anterior vaginal wall testing was completed in subjects with symptoms
[12]. of incontinence. As a method of characterizing
J Sex Med 2006;3:979–987
Nerve Distribution in the Vagina 981
the population, the Female Sexual Function Index Vaginal biopsy specimens were fixed in 10% neu-
(FSFI) was completed by all the subjects preoper- tral buffered formalin and embedded in paraffin
atively. Institutional Review Board approval was blocks using conventional histopathologic tech-
obtained for this study. niques. Ten horizontal 5-mcm-thick levels were
The FSFI is a brief instrument for assessment obtained from each block. Multiple levels were
of sexual function that consists of 19 questions and obtained in an attempt to reduce sampling error
has been validated based on DSM-IV diagnoses of and ensure that the maximum number of nerves
desire disorder, arousal disorder, and orgasmic was generated from each specimen. Levels 1, 3,
dysfunction [22,23]. Questions are scored for and 6 were prepared with hematoxylin and
domains of libido, arousal, lubrication, orgasm, eosin stain for routine microscopy. Levels 2 and 5
satisfaction, and pain. Although other validated were processed for immunohistochemistry accord-
questionnaires are available and some validated for ing to the avidin-biotin-peroxidase complex
use in subjects with prolapse and/or incontinence method using the Ventana Benchmark-automated
[24], we chose the FSFI because it is relatively immunostainer (Ventana Medical Systems, Tucson,
short, is part of our initial patient history forms, AZ) and antibodies against S100 protein (Pre dilute;
and has been utilized by others in studies of this Ventana Medical Systems). A negative control
population [25]. For this study, female sexual dys- (omission of the primary antibody) and a positive
function (FSD) was defined as a total score of 26 control (a Schwannoma) were carried out with each
or less (maximum possible score of 36) [26]. In immunohistochemical reaction. Vaginal smears
those subjects with a total score of 26 or less, were fixed in ThinPrep solution and processed with
additional information was obtained from their the ThinPrep 2000 Processor (Cytyc Corp., Box-
chart to strengthen the diagnosis of sexual dys- borough, MA). The tissue was processed in only
function. In all cases, subjects noted problems with two batches in order to reduce the variances
desire, lubrication, orgasm, or pain elsewhere in between procedures. All tissue processing was per-
their medical history. formed in a clinical lab in conjunction with routine
Prior to surgical draping, a vaginal smear was histology cases. The lab has a daily quality assurance
obtained for maturation index. Maturation index program as guided by the College of American
specimens were taken from the posterior fornix Pathologists. All specimens were evaluated concur-
and labeled with subject identifier. rently by two investigators, one of whom is a Board-
Full-thickness biopsies were obtained in a stan- Certified Pathologist, using a double-headed
dardized manner during surgical repair by one of Olympus BX41 microscope (Center Valley, PA).
five investigators. To ensure good sampling tech- Maturation index cytology specimens were
nique and adequacy of the stains, specimens were evaluated under high (40×) and low (10×) power
initially taken from three cadaver specimens and using multiple fields. Subjects with 40% or more
analyzed in the same fashion. Six potential biopsy superficial cells were classified as having premeno-
sites were identified: proximal and distal anterior pausal estrogenization, 10–39% superficial cells
wall, proximal and distal posterior wall, apex, and were termed intermediate, and those with 9% or
cervix. These sites were chosen to maximize sam- fewer superficial cells in conjunction with para-
pling of the entire vagina. Distal specimens were basal cells were considered to have no estrogen
taken 3 cm inside the hymenal ring, and proximal effect [27].
specimens halfway between the hymen and vaginal S100 immunoperoxidase is a general nerve stain
cuff. Apical specimens were from the vaginal cuff that has been used in studies of heart, gut, and
in patients without a uterus and from the vagina vagina [28–30]. In an effort to ensure that the
adjacent to the cervix in subjects undergoing a maximum nerve estimate was generated for each
hysterectomy. Ectocervical tissue was obtained specimen, a methodology for counting nerves was
after the specimen had been assessed by the devised. Both S100 immunostained levels were
pathology department and was determined to be examined under low power to obtain a general
free of malignancy. The specimens were taken description and locate five fields with the greatest
from the midline after colporrhaphy incisions number of nerves. If required, the hematoxylin
were made, but prior to lateral dissection, using and eosin stains were also utilized to characterize
Mayo scissors. All specimens were color coded for nerve location. Under high power, these 10 fields
location and labeled with subject identifier. The were evaluated and the nerves quantified. Both
majority had five biopsies with a range of 3–6; 110 longitudinal cross sections and radially oriented
biopsies were obtained as described. nerves were counted. It was immediately evident
J Sex Med 2006;3:979–987
982 Pauls et al.
Table 1 Demographic characteristics (N = 21)
Age: mean ± SD 50 ± 12.8 years
Race: Caucasian 21 (100%)
Marital status
Married 20 (95%)
Divorced 1 (5%)
Smoker
Yes 1 (5%)
No 20 (95%)
Menopausal status
Premenopausal 10 (48%)
Postmenopausal 11 (52%)
Hormone replacement 10 (91%)
Vaginal maturation index
Premenopausal 6 (29%)
Intermediate 8 (38%)
No estrogen effect 7 (33%)
Previous hysterectomy 9 (43%)
Abdominal 6 (67%)
Vaginal 3 (33%)
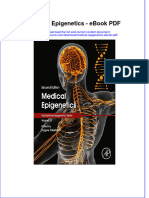
Figure 1 S100 immunostaining; large nerve in center of Previous prolapse repair/anti-incontinence 3 (14%)
photograph, small nerves indicated by arrow. procedure
Stress incontinence 12 (57%)
Fecal incontinence 1 (5%)
Vaginal deliveries: mean 2.7
that there were two types of nerves: small and <3 11 (52%)
large. Because the clinical significance of these two ≥3 10 (48%)
types of nerves was unknown, these were assessed Prolapse stage; maximum descent of any compartment
Stage 2 19 (90%)
separately (see Figure 1). An average number of Stage 3 2 (10%)
large, small, and total nerves per high-power field
were then calculated for each specimen. Excluding
cervical biopsies, one investigator was blinded to
vaginal location of the sample and patient charac- have an anterior and posterior colporrhaphy at the
teristics. The blinded investigator was responsible time of their repair, two opted for nonsurgical
for selection of the fields for quantification. treatments, and one had surgery outside the
Statistical analysis was performed with SPSS approved sites. The remaining 21 subjects com-
13.0 for Windows (SPSS Inc., Chicago, IL). pleted the study (Table 1). The mean age of the
Descriptive statistics, including means and fre- subjects was 50 years. All the subjects were parous
quencies, are reported in Tables 1 and 2. Bivariate and Caucasian. Ten subjects were premenopausal
analyses include independent t-tests, chi-squared with normal monthly menstrual cycles, and 11
test or Fisher exact test, Wilcoxon rank sum test postmenopausal. Twelve patients had urodynamic
(Mann–Whitney test), and Pearson’s r correlation. stress incontinence, whereas the majority had
Effect size calculation was completed using n- stage 2 prolapse. Ten subjects underwent a hyster-
Query Version 6.0 (Statistical Solutions Ltd, Los ectomy at the time of their surgery.
Angeles, CA). Vaginal nerve density was assessed at each ana-
tomic location to compare sites to each other, as
well as to determine the site of highest innervation
Results
(Table 2). One subject was noted to have 250
Twenty-nine patients agreed to participate in this nerves noted at the cuff site only; this specimen
study. Of them, eight were excluded: five did not was excluded from analysis as an outlier. Vaginal
Table 2 Vaginal innervation by location and comparisons of anatomic sites
Mean number Comparison of mean number
Location Subject N of nerves ±SD of nerves at location sites
Distal anterior (DA) 21 21.22 ±10.34 PP > DP > DA > PA > Cervix > Cuff
Proximal anterior (PA) 19 21.19 ±7.30
Distal posterior (DP) 21 21.61 ±9.94
Proximal posterior (PP) 20 24.7 ±6.22
Cuff 18 18.21 ±8.12
Cervix 10 22.45 ±12.75
J Sex Med 2006;3:979–987
Nerve Distribution in the Vagina 983
innervation was relatively uniform, with nerves Table 3 Mean FSFI domain scores (N = 21)
noted throughout the vagina. Overall, the vaginal FSD No FSD
cuff had the least innervation. Sites of the vagina Domain (N = 12) (N = 9) P value
were then compared in each subject who had sam- Desire 2.65 4.13 0.004**
ples taken from all the five vaginal sites (N = 17). Arousal 2.85 4.70 0.002**
There was no site in the vagina that consistently Lubrication 3.48 5.23 0.026*
Orgasm 3.43 4.84 0.185
demonstrated the most innervation: four (23%) Satisfaction 3.10 5.29 0.001**
had the highest innervation in the distal anterior Pain 2.73 5.87 0.000***
vagina, three (18%) had the most nerves at the Total score 18.24 30.07 0.000***
proximal anterior specimen, three (18%) at the *Indicates significance at 0.05 using Wilcoxon rank sum test; **indicates sig-
nificance at 0.01 using Wilcoxon rank sum test; ***indicates significance at
distal posterior, one (6%) subject had the highest 0.001 using Wilcoxon rank sum test.
nerve density at the cuff, and proximal posterior FSFI = Female Sexual Function Index; FSD = female sexual dysfunction.
specimens bore the greatest number of nerves in
six (35%) subjects. Innervation was then assessed with respect to
An overall description of the nerves and their the patient’s sexual function. There was no corre-
location was documented for all specimens. Small lation between total nerves and desire (r = 0.092,
nerves were noted to be thinner and occurred P = 0.691), arousal (r = 0.131, P = 0.571), lubrica-
in each field, whereas large nerves were thicker, tion (r = 0.223, P = 0.332), orgasm (r = 0.121,
often appeared bundled, and were less abundant P = 0.602), satisfaction (r = 0.163, P = 0.480), pain
(Figure 1). There were no nerves noted in the (r = −0.069, P = 0.768), or overall (r = 0.127, P =
epithelium. However, nerves appeared in both the 0.584) scores. Large, small, and total nerves were
superficial and deep submucosa, with relatively subsequently analyzed in subjects with FSD com-
equal distribution. Perivascular innervation, often pared to subjects without (Figures 2 and 3). There
in the deep submucosa, was noted in all anatomic were no differences in total or site-specific nerves
sites. Large nerves tended to be located in the between the two groups.
deeper submucosa, whereas the small nerves were
noted both superficially and deep. Few locore-
Discussion
gional differences were noted except for cervical
specimens. At this site, the majority of the nerves The primary purpose of this study was to charac-
were in the deep submucosa. terize vaginal innervation in women using a large
Nerve quantity and distribution were then sample size, age range, and multiple vaginal biop-
examined in association with the subject’s demo- sies. Previous research has been restricted by these
graphic and medical history. There were no dif- factors, or the use of animal or fetal specimens,
ferences in nerve quantity based on age (P = leading to a limited understanding of nerve distri-
0.910), maturation index (P = 0.901), previous bution in the adult vagina. Whereas studies have
hysterectomy (P = 0.660), or number of vaginal suggested presence of nerves in the distal vagina
deliveries (P = 0.299). The total number of nerves [11], there is a perception that the upper vagina is
was similar based on stage of prolapse (P = 0.384) deplete in innervation. We report consistent nerve
and in those with stress urinary incontinence (P = distribution throughout the vagina, with the vag-
0.900). Nerve location was then assessed based on inal cuff showing slightly fewer nerves than the
subject age, maturation index, menopausal status, anterior and posterior walls. This distribution
previous hysterectomy, parity, stage of prolapse, could be attributed to the embryological origin of
and stress incontinence. There were no significant these structures, with the vagina derived from the
relationships noted between depth of nerves or urogenital sinus bearing greater nerves than that
perivascular innervation and these factors. of the müllerian ducts.
Twelve (57%) subjects had FSD based on the What remains uncertain is the purpose of the
FSFI. Total and domain scores were significantly vaginal nerves we described. Vaginal eroticism has
different in subjects with dysfunction compared been reported, with stimulation of both anterior
to those without in all domains except orgasm and posterior vagina leading to orgasm [14,15].
(Table 3). Comparison of demographic informa- Central and peripheral neurological disorders lead
tion was performed for subjects with and without to problems with orgasm, genital sensation,
dysfunction. Age was the only factor that was decreased lubrication, and sexual desire [19],
significant; younger age was associated with FSD implying that adequate innervation is important to
(P = 0.012). these functions. New studies on mechanisms of
J Sex Med 2006;3:979–987
984 Pauls et al.
3.5
P = .208
3.0
P = .338
2.5
Mean Nerves
P = .906
2.0
P = .394
P = .827
1.5
P = .564
1.0
0.5
P = .348
0.0
e
ge
s
ite
rg
rg
rg
rg
rg
ar
La
La
La
La
La
lS
fL
Al
r
x
uf
rio
rio
io
io
vi
of
C
er
er
er
te
te
st
st
e
An
An
g
Po
Po
Figure 2 Mean large nerves in
ra
l
al
ta
e
l
al
im
ta
Av
is
im
is
patients with FSD vs. no FSD.
D
ox
e
D
ox
rg
Pr
Pr
FSD = female sexual dysfunction.
FSD No FSD
arousal show that central input is important in La ween groups. Therefore, we postulate that alth-
blood flow changes to the vagina [31], and ana- ough immunohistochemical staining yields a
tomic studies suggest a role for innervation in vag- description of nerve density, it does not provide
inal blood flow and capillary permeability [32]. information of nerve behavior. Nerve function
Finally, a recent study using the rat model dem- may be different in subjects with FSD compared
onstrated a link between the vagina and central to those without. Unfortunately, vaginal nerve
nervous system sites involved in female sexual activity is not easily assessed using conventional
responses [33]. We hypothesized that sexual surface electromyogram or pudendal nerve motor
responses, such as arousal, lubrication, orgasm, latency methodology.
and pain, may be mediated by nerve fibers termi- Previous anecdotal evidence suggests the exist-
nating in the vaginal mucosa, and consequently ence of an erogenous zone on the anterior vaginal
the nerve number would be associated with sexual wall at the bladder neck, termed the “Gräfenberg,”
dysfunction. However, we report no differences in or “G-spot” [16,35]. Presence of such an area has
total or site-specific innervation in patients with been widely accepted by the general public; how-
dysfunction compared to those without. A post- ever, anatomic proof for this site is inconclusive
hoc effect size calculation was performed and [36,37]. Research has suggested that the paraure-
revealed a large effect of 2.18 [34]. While this thral glands are the homolog to the male prostate
result could be biased due to our small sample, and [38], and some think these glands may be contrib-
therefore should be interpreted with caution, it utory to this zone, whereas others feel that it is the
suggests that the results are supported by adequate bulbs of the clitoris [39] draping down on the
power to determine significant differences bet- anterior vagina that leads to eroticism at this site.
P = .088
P = .466
25 P = .558
P = .951 P = .983
P = .937 P = .829
P = .757
20
Mean Nerves
15
10
0
l
s
al
al
al
al
al
al
it e
ite
m
Sm
lS
lS
rS
rS
rS
rS
fS
Al
Al
x
uf
io
io
rio
rio
vi
of
of
C
er
er
te
te
te
nt
ge
ge
An
os
os
lA
ra
ra
lP
P
al
ta
ve
Figure 3 Mean small and total nerves
al
m
ta
Av
is
im
lA
xi
is
D
s
ox
al
ve
Pr
Sm
in patients with FSD vs. no FSD.
Pr
er
lN
FSD No FSD
FSD = female sexual dysfunction.
ta
To
J Sex Med 2006;3:979–987
Nerve Distribution in the Vagina 985
We did not document a corresponding increase in bias. Finally, it is important to note that nerves
innervation in the anterior vagina. However, we on histology may not represent type and mode
do not claim that this is proof that the G-spot does of tissue innervation. It is not possible to know
not exist. Our study did not specifically set out to from immunohistochemical stains the nature of
find the G-spot; we were limited by a single biopsy an organ’s gross innervation, which limits the
in the distal anterior vagina and only sampling findings described here.
vaginal mucosa. Thus it is possible that we may Nerve quantity alone cannot be predictive
have missed this area. of sexual function or dysfunction. While we
A secondary aim of this study was to quantify explored a potential relationship between sexual
innervation based on various demographic factors, function and nerve density, we were aware that this
including vaginal estrogenization and previous is only one factor. Sexual function is complex,
hysterectomy. There are conflicting reports about impacted by multiple issues in the context of a
the effects of estrogen on nerve size [4,6,8,32], woman’s life, and many variables may have been
with a recent study showing that testosterone responsible for the differences seen here. Never-
treatment, rather than estrogen, led to increases in theless, our findings have advanced our knowledge
vaginal nerve fibers [8]. We did not note a differ- regarding vaginal anatomy and sexuality. Future
ence in innervation based on estrogen status in the investigation should attempt to assess nerve
vagina, similar to other reports [6,8,32]. This sug- behavior in relation to sexual function, rather than
gests that estrogen may not be a mediator of nerve nerve density.
quantity or size in the vagina. Recently, there has
been interest in development of surgical tech- Conclusions
niques to preserve pelvic autonomic nerves and
reduce potential negative impacts of hysterectomy In summary, a prospective study in women involv-
on sexual function [19]. We found no difference in ing biopsies of proximal and distal anterior and
nerve quantity or distribution in our subjects who posterior portions of the vagina, including apex
had undergone prior hysterectomy. Although our and cervix, was performed. Vaginal innervation
findings cannot predict function or type of inner- was regularly identified in all locations, with no
vation, vaginal nerve quantity and location appears site of increased density. There was no correlation
to be unaffected by hysterectomy. Further studies between vaginal nerve quantity and the patient’s
are needed to establish the impact of hysterectomy demographic information or sexual function.
on pelvic nerve and sexual function.
One limitation of our work is that all the sub- Acknowledgments
jects had prolapse. Prolapse has been associated
The author (R.P.) would like to acknowledge the Tri-
with subjectively decreased vaginal innervation
Health Hatton Research Department for providing
[28], making this a potential confounder. Al- funding for this project.
though we did not note a significant difference
in our subjects based on stage of prolapse, it is Corresponding Author: Rachel Pauls, MD, Good
possible that subtle changes were present in Samaritan Hospital—Division of Urogynecology and
these specimens. We were aware of this limita- Reconstructive Pelvic Surgery, 375 Dixmyth Ave
tion, but thought that this was the best way to Seton Center, 8th Floor, Cincinnati, OH 45220, USA.
obtain multiple biopsies from the vagina with Tel: +1-513-872-1658; Fax: +1-513-872-4498; E-mail:
minimal risk to the patient. Because patients rachel_pauls@trihealth.com
with prolapse constituted the entire sample, it Conflict of Interest: None declared.
was felt that comparisons within and between
subjects with respect to sexual function and
References
nerve location would be acceptable. A second
limitation exists in the use of histological sec- 1 Croak AJ, Gebhart JB, Klingele CJ, Lee RA, Ray-
tions to quantify nerve number. Although mea- burn WF. Therapeutic strategies for vaginal Mulle-
rian agenesis. J Reprod Med 2003;48:395–401.
sures were taken to maximize the number of
2 Capenter SEK, Rock JA. Pediatric and adolescent
nerves estimated from each biopsy, by selecting gynecology, 2nd edn. Lippincott: Williams &
only the 10 highest density fields for analysis, we Wilkins; 2000.
may have misrepresented the true density of the 3 Sobel V, Zhu YS, Imperato-McGinley J. Fetal hor-
specimen. Random selection of fields for quanti- mones and sexual differentiation. Obstet Gynecol
fication may be a means to reduce this potential Clin North Am 2004;31:837–56 x–xi.
J Sex Med 2006;3:979–987
986 Pauls et al.
4 McKenna KE. The neurophysiology of female sex- tion using noninvasive techniques. Am J Obstet
ual function. World J Urol 2002;20:93–100. Gynecol 2005;192:1712–7.
5 Giraldi A, Marson L, Nappi R, Pfaus J, Traish AM, 21 Bump RC, Mattiasson A, Bo K, Brubaker LP,
Vardi Y, Goldstein I. Physiology of female sexual DeLancey JO, Klarskov P, Shull BL, Smith AR.
function: Animal models. J Sex Med 2004;1:237–53. The standardization of terminology of female pelvic
6 Giraldi A, Alm P, Werkstrom V, Myllymaki L, organ prolapse and pelvic floor dysfunction. Am J
Wagner G, Andersson KE. Morphological and Obstet Gynecol 1996;175:10–7.
functional characterization of a rat vaginal smooth 22 Meston CM. Validation of the Female Sexual Func-
muscle sphincter. Int J Impot Res 2002;14:271–82. tion Index (FSFI) in women with female orgasmic
7 Onol FF, Ercan F, Tarcan T. The effect of ovariec- disorder and in women with hypoactive sexual
tomy on rat vaginal tissue contractility and histo- desire disorder. J Sex Marital Ther 2003;29:39–46.
morphology. J Sex Med 2006;3:233–41. 23 Rosen R, Brown C, Heiman J, Leiblum S, Meston
8 Pessina MA, Hoyt RF Jr, Goldstein I, Traish AM. C, Shabsigh R, Ferguson D, D’Agostino R Jr. The
Differential effects of estradiol, progesterone, and Female Sexual Function Index (FSFI): A multidi-
testosterone on vaginal structural integrity. Endo- mensional self-report instrument for the assessment
crinology 2006;147:61–9. of female sexual function. J Sex Marital Ther
9 Krantz KE. Innervation of the human vulva and 2000;26:191–208.
vagina; A microscopic study. Obstet Gynecol 1958; 24 Rogers RG, Kammerer-Doak D, Villarreal A,
12:382–96. Coates K, Qualls C. A new instrument to measure
10 Owman C, Rosenbren E, Sjoberg NO. Adrenergic sexual function in women with urinary incontinence
innervation of the human female reproductive or pelvic organ prolapse. Am J Obstet Gynecol
organs: A histochemical and chemical investigation. 2001;184:552–8.
Obstet Gynecol 1967;30:763–73. 25 Salonia A, Zanni G, Nappi RE, Briganti A, Deho F,
11 Hilliges M, Falconer C, Ekman-Ordeberg G, Fabbri F, Colombo R, Guazzoni G, DiGirolamo V,
Johansson O. Innervation of the human vaginal Rigatti P, Montorsi F. Sexual dysfunction is com-
mucosa as revealed by PGP 9.5 immunohistochem- mon in women with lower urinary tract symptoms
istry. Acta Anat (Basel) 1995;153:119–26. and urinary incontinence: Results of a cross-
12 Yucel S, De SA Jr, Baskin LS. Neuroanatomy of sectional study. Eur Urol 2004;45:642–8.
the human female lower urogenital tract. J Urol 26 Wiegel M, Meston C, Rosen R. The female sexual
2004;172:191–5. function index (FSFI): Cross-validation and devel-
13 Stjernholm Y, Sennstrom M, Granstrom L, Ekman opment of clinical cutoff scores. J Sex Marital Ther
G, Johansson O. Protein gene product 9.5-immu- 2005;31:1–20.
noreactive nerve fibers and cells in human cervix of 27 DeMay RM. The art and science of cytopathology.
late pregnant, postpartal and non-pregnant women. Chicago: American Society of Clinical Pathologists,
Acta Obstet Gynecol Scand 1999;78:299–304. 1996.
14 Alzate H, Londono ML. Vaginal erotic sensitivity. 28 Boreham MK, Wai CY, Miller RT, Schaffer JI,
J Sex Marital Ther 1984;10:49–56. Word RA. Morphometric properties of the poste-
15 Alzate H. Vaginal eroticism: A replication study. rior vaginal wall in women with pelvic organ pro-
Arch Sex Behav 1985;14:529–37. lapse. Am J Obstet Gynecol 2002;187:1501–8.
16 Goldberg DC, Whipple B, Fishkin RE, Waxman H, 29 Nemeth L, Puri P. The innervation of human bowel
Fink PJ, Weisberg M. The Grafenberg spot and mucosa and its alterations in Hirschsprung’s disease
female ejaculation: A review of initial hypotheses. J using a whole-mount preparation technique. Pedi-
Sex Marital Ther 1983;9:27–37. atr Surg Int 2000;16:277–81.
17 Shafik A, El Sibai O, Shafik AA, Ahmed I, Mostafa 30 Park AM, Armin S, Azarbal A, Lai A, Chen PS,
RM. The electrovaginogram: Study of the vaginal Fishbein MC. Distribution of cardiac nerves in
electric activity and its role in the sexual act and patients with diabetes mellitus: An immunohis-
disorders. Arch Gynecol Obstet 2004;269:282–6. tochemical postmortem study of human hearts.
18 Basson R, Leiblum S, Brotto L, Derogatis L, Four- Cardiovasc Pathol 2002;11:326–31.
croy J, Fugl-Meyer K, Graziottin A, Heiman JR, 31 Brotto LA, Basson R, Gorzalka BB. Psychophysio-
Laan E, Meston C, Shover L, vanLankveld J, logical assessment in premenopausal sexual arousal
Schultz WW. Revised definitions of women’s sexual disorder. J Sex Med 2004;1:266–77.
dysfunction. J Sex Med 2004;1:40–8. 32 Hoyle CH, Stones RW, Robson T, Whitley K,
19 Nappi R, Salonia A, Traish AM, van Lunsen RH, Burnstock G. Innervation of vasculature and
Vardi Y, Kodiglu A, Goldstein I. Clinical biologic microvasculature of the human vagina by NOS and
pathophysiologies of women’s sexual dysfunction. J neuropeptide-containing nerves. J Anat 1996;188:
Sex Med 2005;2:4–25. 633–44.
20 Connell K, Guess MK, La Combe J, Wang A, Pow- 33 Marson L, Murphy AZ. Identification of neural cir-
ers K, Lazarou G, Mikhail M. Evaluation of the role cuits involved in female genital responses in the rat:
of pudendal nerve integrity in female sexual func- A dual virus and anterograde tracing study. Am J
J Sex Med 2006;3:979–987
Nerve Distribution in the Vagina 987
Physiol Regul Integr Comp Physiol 2006;29(2):19– 37 Hines TM. The G-spot: A modern gyneco-
28. logic myth. Am J Obstet Gynecol 2001;185:
34 Cohen J. Statistical power analysis for the behavioral 359–62.
sciences, 2nd edn. Hillsdale, NJ: Lawrence Earl- 38 Zaviacic M, Ablin RJ. The female prostate and pros-
baum Associates; 1988. Epub February 16, 2006. tate-specific antigen. Immunohistochemical local-
35 Addiego F, Belzer E, Comolli J, Moger W, Perry J, ization, implications of this prostate marker in
Whipple B. Female ejaculation: A case study. J Sex women and reasons for using the term “prostate” in
Res 1981;17:13–21. the human female. Histol Histopathol 2000;15:131–
36 Alzate H, Hoch Z. The “G spot” and “female ejac- 42.
ulation”: A current appraisal. J Sex Marital Ther 39 O’Connell HE, Sanjeevan KV, Hutson JM. Anat-
1986;12:211–20. omy of the clitoris. J Urol 2005;174:1189–95.
J Sex Med 2006;3:979–987
You might also like
- The Maternal OrganismFrom EverandThe Maternal OrganismN. S. AssaliNo ratings yet
- Review of Prostate Anatomy and Embryology and The Etiology of Benign Prostatic Hyperplasia PDFDocument10 pagesReview of Prostate Anatomy and Embryology and The Etiology of Benign Prostatic Hyperplasia PDFAlejandro GuerreroNo ratings yet
- Prostate-Induced Orgasms:: A Concise Review Illustrated With A Highly Relevant Case StudyDocument5 pagesProstate-Induced Orgasms:: A Concise Review Illustrated With A Highly Relevant Case StudyDayanaNo ratings yet
- Ambiguous Genitalia 3Document4 pagesAmbiguous Genitalia 3syarifah salmaNo ratings yet
- The Female Prostate - The Newly Recognized Organ of The Female Genitourinary SystemDocument15 pagesThe Female Prostate - The Newly Recognized Organ of The Female Genitourinary SystemAlberto01100% (1)
- Neurobiology of Sexual FunctionDocument20 pagesNeurobiology of Sexual FunctionLisbel Durán ParradoNo ratings yet
- Accessory Urethra, Accessory PhallusDocument7 pagesAccessory Urethra, Accessory PhallusGunduz AgaNo ratings yet
- Method of Physiological Evaluating Sexual FunctionDocument16 pagesMethod of Physiological Evaluating Sexual Functiondeargrace2512No ratings yet
- McKenna (2021) Arch Sex Behav Trigger For Sexual ClimaxDocument8 pagesMcKenna (2021) Arch Sex Behav Trigger For Sexual ClimaxPetr Šupa100% (1)
- Control Neuronal de Los Comportamientos Sexuales FemeninosDocument11 pagesControl Neuronal de Los Comportamientos Sexuales FemeninosDORIS JANETHNo ratings yet
- Skenes Gland Revisited Function Dysfunction and TDocument4 pagesSkenes Gland Revisited Function Dysfunction and TNexi anessaNo ratings yet
- Safe Female CircumcisionDocument51 pagesSafe Female Circumcisiond7rqqrk+vaii68100% (1)
- Laporan Tutorial1-Fbs1 ( (Bayi Tabung) )Document80 pagesLaporan Tutorial1-Fbs1 ( (Bayi Tabung) )kirei_thaNo ratings yet
- Email: Fcabello@ingenia - Es: Cabello Santa Maria Paco - Spain CABRAL Blanca E - VenezuelaDocument20 pagesEmail: Fcabello@ingenia - Es: Cabello Santa Maria Paco - Spain CABRAL Blanca E - VenezuelaWulan CerankNo ratings yet
- 2000 - Hypospadias. Anatomy, Embryology, andDocument9 pages2000 - Hypospadias. Anatomy, Embryology, andtiaraNo ratings yet
- Nihms 776474Document17 pagesNihms 776474Antares Basulto NathNo ratings yet
- Del 022Document5 pagesDel 022BattleAppleNo ratings yet
- Accepted Manuscript: UrologyDocument7 pagesAccepted Manuscript: UrologyTeja LaksanaNo ratings yet
- Sexual Medicine ReviewsDocument14 pagesSexual Medicine ReviewsilhamNo ratings yet
- HHS Public Access: Association of Cervical Effacement With The Rate of Cervical Change in Labor Among Nulliparous WomenDocument12 pagesHHS Public Access: Association of Cervical Effacement With The Rate of Cervical Change in Labor Among Nulliparous WomenM Iqbal EffendiNo ratings yet
- Ultrasound Assessment of The Polycystic Ovary: International Consensus De®nitionsDocument10 pagesUltrasound Assessment of The Polycystic Ovary: International Consensus De®nitionsArkhan HanafiNo ratings yet
- 10 1016@j Ajog 2019 07 010 PDFDocument13 pages10 1016@j Ajog 2019 07 010 PDFDaniel GamarraNo ratings yet
- Toksemia GravidarumDocument9 pagesToksemia GravidarumabdullahNo ratings yet
- Anal and Ano-Urogenital Malformations: A Histopathological Study of "Imperforate Anus" With A Reconstruction of The PathogenesisDocument7 pagesAnal and Ano-Urogenital Malformations: A Histopathological Study of "Imperforate Anus" With A Reconstruction of The PathogenesisTuti DamNo ratings yet
- Case Study-Fibroids in PregnancyDocument10 pagesCase Study-Fibroids in Pregnancysimbarashe tangwadzanaNo ratings yet
- Skene's Gland Revisited - Function, Dysfunction and The G SpotDocument3 pagesSkene's Gland Revisited - Function, Dysfunction and The G SpotVettyBNo ratings yet
- Author Information Kafaei Atrian MDocument9 pagesAuthor Information Kafaei Atrian MabdullahNo ratings yet
- The Neurobiology of Sexual FunctionDocument19 pagesThe Neurobiology of Sexual FunctionкостяNo ratings yet
- J Ajog 2008 10 040Document5 pagesJ Ajog 2008 10 040José Vivas EspinozaNo ratings yet
- 1 s2.0 S0140673610603558 MainDocument11 pages1 s2.0 S0140673610603558 MainDr. Eser AĞARNo ratings yet
- Perspectives: Beyond The G Spot: Clitourethrovaginal Complex Anatomy in Female OrgasmDocument8 pagesPerspectives: Beyond The G Spot: Clitourethrovaginal Complex Anatomy in Female OrgasmVissente TapiaNo ratings yet
- University Journal of Surgery and Surgical Specialities: Dhivya Lakshmi S JDocument4 pagesUniversity Journal of Surgery and Surgical Specialities: Dhivya Lakshmi S JYonathanWaisendiNo ratings yet
- J Urology 2007 06 842Document1 pageJ Urology 2007 06 842quickdannyNo ratings yet
- Measurements of A Normal Vulva' in Women Aged 15-84 - A Cross Sectional Prospective Single Centre StudyDocument6 pagesMeasurements of A Normal Vulva' in Women Aged 15-84 - A Cross Sectional Prospective Single Centre StudySika JantunenNo ratings yet
- Ajacm 2009 4 (1) Acup Improve EndometrDocument7 pagesAjacm 2009 4 (1) Acup Improve EndometrdirkvandeweyerNo ratings yet
- Pediatric Urology PDFDocument14 pagesPediatric Urology PDFekalospratamaNo ratings yet
- Author Information Kafaei Atrian MDocument9 pagesAuthor Information Kafaei Atrian MabdullahNo ratings yet
- Scaramuzzi 2011 PDFDocument24 pagesScaramuzzi 2011 PDFKarol Mariam VelázquezNo ratings yet
- Does Female Ejaculation Serve An Antimicrobial PurposeDocument3 pagesDoes Female Ejaculation Serve An Antimicrobial PurposeJulian Ricardo Corrales FierroNo ratings yet
- The Female Prostate Revisited: Perineal Ultrasound and Biochemical Studies of Female EjaculateDocument6 pagesThe Female Prostate Revisited: Perineal Ultrasound and Biochemical Studies of Female EjaculatephampullovNo ratings yet
- Hymen ImperforataDocument16 pagesHymen ImperforataImanuella KairupanNo ratings yet
- Kamasutra in Practice The Use of Sexual Positions in The CzechDocument10 pagesKamasutra in Practice The Use of Sexual Positions in The CzechYoga PrabawaNo ratings yet
- Parurethral CystsDocument3 pagesParurethral CystsIoannis ValioulisNo ratings yet
- Poor Spontaneous and Oxytocin-Stimulated Contractility in Human Myometrium From Postdates PregnanciesDocument11 pagesPoor Spontaneous and Oxytocin-Stimulated Contractility in Human Myometrium From Postdates PregnanciesLauren RenNo ratings yet
- Sex Redefined - Revista NatureDocument4 pagesSex Redefined - Revista NatureWilliam Harris100% (1)
- Jurnal Kedokteran ObgynDocument6 pagesJurnal Kedokteran Obgynfa_ndriNo ratings yet
- Fertility PreservationDocument9 pagesFertility PreservationAnizkha MitternachtNo ratings yet
- Male RSDocument7 pagesMale RSpravina praviNo ratings yet
- TestosteronaDocument7 pagesTestosteronaTomás DíazNo ratings yet
- Physiology of Womens Sexual Function BasDocument24 pagesPhysiology of Womens Sexual Function BasкостяNo ratings yet
- Undescended Testes:Embryological and Clinical Importance: Veena Vidya Shankar, Roopa KulkarniDocument3 pagesUndescended Testes:Embryological and Clinical Importance: Veena Vidya Shankar, Roopa KulkarniArgy RizkyNo ratings yet
- 45 HypospadiaDocument50 pages45 HypospadiaMarcella SaputraNo ratings yet
- The Neural and Genetic Correlates of Satisfying Sexual Activity in Heterosexual Pair BondsDocument16 pagesThe Neural and Genetic Correlates of Satisfying Sexual Activity in Heterosexual Pair BondsClaudia Cuautle RamirezNo ratings yet
- Refarat Ginekologi - Prolaps Organ PanggulDocument29 pagesRefarat Ginekologi - Prolaps Organ Panggullhia priscillaNo ratings yet
- Differences Between Male and Female Sexual FunctioningDocument4 pagesDifferences Between Male and Female Sexual FunctioningaleksasNo ratings yet
- Prenatal Diagnosis of Sex Differentiation DisorderDocument8 pagesPrenatal Diagnosis of Sex Differentiation DisorderHorvath AlexandraNo ratings yet
- Current Insights Sperm Movility 2022Document19 pagesCurrent Insights Sperm Movility 2022Diego De La TorreNo ratings yet
- The Accuracy of 2D Ultrasound Prenatal Sex Determination: Original ArticleDocument6 pagesThe Accuracy of 2D Ultrasound Prenatal Sex Determination: Original ArticledrIndah NoviantyNo ratings yet
- Vol32 n4 2006 PDFDocument115 pagesVol32 n4 2006 PDFNilberto NascimentoNo ratings yet
- Fine-Touch Pressure Thresholds in The Adult PenisDocument6 pagesFine-Touch Pressure Thresholds in The Adult Penissmashzionism12345No ratings yet
- Chylothorax OriginalDocument4 pagesChylothorax OriginalMaria CostandachiNo ratings yet
- (515466942) 274905Document42 pages(515466942) 274905Anonymous JXKgWBjerNo ratings yet
- Egurukul OrbitDocument8 pagesEgurukul OrbitbetsyNo ratings yet
- Musculoskeletal ExaminationDocument42 pagesMusculoskeletal ExaminationRahulNo ratings yet
- Dumitrita Rodica Bagireanu U4 Assessment Feedback2 (PASS)Document4 pagesDumitrita Rodica Bagireanu U4 Assessment Feedback2 (PASS)Rodica Dumitrita0% (1)
- AW32150 - 30 - Surgical Guideline SYNCHRONY PIN - EN English - WebDocument64 pagesAW32150 - 30 - Surgical Guideline SYNCHRONY PIN - EN English - WebLong An DoNo ratings yet
- Family Planning in The Hospital Operational Guide For Recording and Reporting PDFDocument69 pagesFamily Planning in The Hospital Operational Guide For Recording and Reporting PDFViolet CherryNo ratings yet
- EUOGS OSCE Booklet 2020Document26 pagesEUOGS OSCE Booklet 2020Amanda Leow100% (1)
- The Influence of Probiotics and Antibiotic Growth PromoterDocument7 pagesThe Influence of Probiotics and Antibiotic Growth PromoterOliver TalipNo ratings yet
- KN 4@enzl 8 Ha 4 B 6 CC 9 eDocument26 pagesKN 4@enzl 8 Ha 4 B 6 CC 9 eRamzen Raphael DomingoNo ratings yet
- Diagnostic Approach To The Adult With Jaundice or Asymptomatic Hyperbilirubinemia - UpToDateDocument17 pagesDiagnostic Approach To The Adult With Jaundice or Asymptomatic Hyperbilirubinemia - UpToDateVictor MarquesNo ratings yet
- Full Download Book Medical Epigenetics PDFDocument41 pagesFull Download Book Medical Epigenetics PDFandrew.lindsey981100% (14)
- Centrilobular Fibrosis in Fibrotic (Chronic)Document8 pagesCentrilobular Fibrosis in Fibrotic (Chronic)yuriescaidaNo ratings yet
- 2019 Jerrold The Extraction of Teeth Part 1 Considerations Regarding Which Teeth To Extract Seminars in OrthodonticsDocument5 pages2019 Jerrold The Extraction of Teeth Part 1 Considerations Regarding Which Teeth To Extract Seminars in Orthodonticsplayer osama100% (1)
- Medical AbbreviationsDocument31 pagesMedical Abbreviationsrekammedis lddlNo ratings yet
- General AnaestheticDocument10 pagesGeneral AnaestheticFeyaNo ratings yet
- Short Increment Sensitivity Index Test (SISI)Document2 pagesShort Increment Sensitivity Index Test (SISI)Anish RajNo ratings yet
- Fynadine 20mgDocument2 pagesFynadine 20mgNgo Van TruongNo ratings yet
- Ketidakseimbangan Elektrolit (Corat Coret)Document5 pagesKetidakseimbangan Elektrolit (Corat Coret)jendal_kancilNo ratings yet
- Naturopathic Nutrition: Non-Vitamin / Mineral SupplementsDocument27 pagesNaturopathic Nutrition: Non-Vitamin / Mineral Supplementsglenn johnston100% (1)
- Toxic Epidermal NecrolysisDocument13 pagesToxic Epidermal NecrolysisHend AbdallaNo ratings yet
- Ok Urologi Maret 2021 Minggu IDocument25 pagesOk Urologi Maret 2021 Minggu ILittle DevNo ratings yet
- The Management of Patients With Acute Myocardial Infarction: Pocket GuidelinesDocument22 pagesThe Management of Patients With Acute Myocardial Infarction: Pocket GuidelinesAhmad Yuliandri MustopaNo ratings yet
- Hypertrophic ScarsDocument13 pagesHypertrophic ScarsNovita NingtyasNo ratings yet
- Pem Quiz: Respiratory Emergencies Peter S. Auerbach, MDDocument3 pagesPem Quiz: Respiratory Emergencies Peter S. Auerbach, MDPhanVHNo ratings yet
- Dental BiofilmsDocument44 pagesDental BiofilmsRamona MateiNo ratings yet
- Evaluation of The Painful EyeDocument9 pagesEvaluation of The Painful EyeMuthia Farah AshmaNo ratings yet
- Olive, D. L., & Pritts, E. A. (2001) - Treatment of Endometriosis. The New England Journal of Medicine, 345 (4), 266-275.Document11 pagesOlive, D. L., & Pritts, E. A. (2001) - Treatment of Endometriosis. The New England Journal of Medicine, 345 (4), 266-275.Hernando Rivera-DuqueNo ratings yet
- 2023 Projects Microbiology-Hons-BookletDocument56 pages2023 Projects Microbiology-Hons-Bookletchand198No ratings yet
- Basic Principles of Periodontal SurgeryDocument100 pagesBasic Principles of Periodontal SurgerySandip Ladani100% (2)
- The Obesity Code: Unlocking the Secrets of Weight LossFrom EverandThe Obesity Code: Unlocking the Secrets of Weight LossRating: 4 out of 5 stars4/5 (6)
- The Age of Magical Overthinking: Notes on Modern IrrationalityFrom EverandThe Age of Magical Overthinking: Notes on Modern IrrationalityRating: 4 out of 5 stars4/5 (28)
- By the Time You Read This: The Space between Cheslie's Smile and Mental Illness—Her Story in Her Own WordsFrom EverandBy the Time You Read This: The Space between Cheslie's Smile and Mental Illness—Her Story in Her Own WordsNo ratings yet
- Think This, Not That: 12 Mindshifts to Breakthrough Limiting Beliefs and Become Who You Were Born to BeFrom EverandThink This, Not That: 12 Mindshifts to Breakthrough Limiting Beliefs and Become Who You Were Born to BeRating: 2 out of 5 stars2/5 (1)
- ADHD is Awesome: A Guide to (Mostly) Thriving with ADHDFrom EverandADHD is Awesome: A Guide to (Mostly) Thriving with ADHDRating: 5 out of 5 stars5/5 (1)
- LIT: Life Ignition Tools: Use Nature's Playbook to Energize Your Brain, Spark Ideas, and Ignite ActionFrom EverandLIT: Life Ignition Tools: Use Nature's Playbook to Energize Your Brain, Spark Ideas, and Ignite ActionRating: 4 out of 5 stars4/5 (404)
- Raising Mentally Strong Kids: How to Combine the Power of Neuroscience with Love and Logic to Grow Confident, Kind, Responsible, and Resilient Children and Young AdultsFrom EverandRaising Mentally Strong Kids: How to Combine the Power of Neuroscience with Love and Logic to Grow Confident, Kind, Responsible, and Resilient Children and Young AdultsRating: 5 out of 5 stars5/5 (1)
- Summary: Outlive: The Science and Art of Longevity by Peter Attia MD, With Bill Gifford: Key Takeaways, Summary & AnalysisFrom EverandSummary: Outlive: The Science and Art of Longevity by Peter Attia MD, With Bill Gifford: Key Takeaways, Summary & AnalysisRating: 4.5 out of 5 stars4.5/5 (42)
- Summary: The Psychology of Money: Timeless Lessons on Wealth, Greed, and Happiness by Morgan Housel: Key Takeaways, Summary & Analysis IncludedFrom EverandSummary: The Psychology of Money: Timeless Lessons on Wealth, Greed, and Happiness by Morgan Housel: Key Takeaways, Summary & Analysis IncludedRating: 5 out of 5 stars5/5 (81)
- The Ritual Effect: From Habit to Ritual, Harness the Surprising Power of Everyday ActionsFrom EverandThe Ritual Effect: From Habit to Ritual, Harness the Surprising Power of Everyday ActionsRating: 3.5 out of 5 stars3.5/5 (3)
- Outlive: The Science and Art of Longevity by Peter Attia: Key Takeaways, Summary & AnalysisFrom EverandOutlive: The Science and Art of Longevity by Peter Attia: Key Takeaways, Summary & AnalysisRating: 4 out of 5 stars4/5 (1)
- Why We Die: The New Science of Aging and the Quest for ImmortalityFrom EverandWhy We Die: The New Science of Aging and the Quest for ImmortalityRating: 4 out of 5 stars4/5 (3)
- The Body Keeps the Score by Bessel Van der Kolk, M.D. - Book Summary: Brain, Mind, and Body in the Healing of TraumaFrom EverandThe Body Keeps the Score by Bessel Van der Kolk, M.D. - Book Summary: Brain, Mind, and Body in the Healing of TraumaRating: 4.5 out of 5 stars4.5/5 (266)
- Sleep Stories for Adults: Overcome Insomnia and Find a Peaceful AwakeningFrom EverandSleep Stories for Adults: Overcome Insomnia and Find a Peaceful AwakeningRating: 4 out of 5 stars4/5 (3)
- Love Life: How to Raise Your Standards, Find Your Person, and Live Happily (No Matter What)From EverandLove Life: How to Raise Your Standards, Find Your Person, and Live Happily (No Matter What)No ratings yet
- When the Body Says No by Gabor Maté: Key Takeaways, Summary & AnalysisFrom EverandWhen the Body Says No by Gabor Maté: Key Takeaways, Summary & AnalysisRating: 3.5 out of 5 stars3.5/5 (2)
- Gut: the new and revised Sunday Times bestsellerFrom EverandGut: the new and revised Sunday Times bestsellerRating: 4 out of 5 stars4/5 (392)
- Cult, A Love Story: Ten Years Inside a Canadian Cult and the Subsequent Long Road of RecoveryFrom EverandCult, A Love Story: Ten Years Inside a Canadian Cult and the Subsequent Long Road of RecoveryRating: 4 out of 5 stars4/5 (44)
- Dark Psychology & Manipulation: Discover How To Analyze People and Master Human Behaviour Using Emotional Influence Techniques, Body Language Secrets, Covert NLP, Speed Reading, and Hypnosis.From EverandDark Psychology & Manipulation: Discover How To Analyze People and Master Human Behaviour Using Emotional Influence Techniques, Body Language Secrets, Covert NLP, Speed Reading, and Hypnosis.Rating: 4.5 out of 5 stars4.5/5 (110)
- The Marshmallow Test: Mastering Self-ControlFrom EverandThe Marshmallow Test: Mastering Self-ControlRating: 4.5 out of 5 stars4.5/5 (58)
- Mindset by Carol S. Dweck - Book Summary: The New Psychology of SuccessFrom EverandMindset by Carol S. Dweck - Book Summary: The New Psychology of SuccessRating: 4.5 out of 5 stars4.5/5 (328)
- Dark Psychology: Learn To Influence Anyone Using Mind Control, Manipulation And Deception With Secret Techniques Of Dark Persuasion, Undetected Mind Control, Mind Games, Hypnotism And BrainwashingFrom EverandDark Psychology: Learn To Influence Anyone Using Mind Control, Manipulation And Deception With Secret Techniques Of Dark Persuasion, Undetected Mind Control, Mind Games, Hypnotism And BrainwashingRating: 4 out of 5 stars4/5 (1138)
- Troubled: A Memoir of Foster Care, Family, and Social ClassFrom EverandTroubled: A Memoir of Foster Care, Family, and Social ClassRating: 4.5 out of 5 stars4.5/5 (26)