Professional Documents
Culture Documents
Nelson Chemistry 11 Textbook Nelson Education Free Download, Borrow, and Streaming Internet Archive
Uploaded by
assal2007faisalOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Nelson Chemistry 11 Textbook Nelson Education Free Download, Borrow, and Streaming Internet Archive
Uploaded by
assal2007faisalCopyright:
Available Formats
Questions
$ Nelson Chemistry 11 Textbook
1. How do single and double displacement reactions differ?
☆
Favoriteindustry
#
Shareas Flag
⚑
by Nelson Education (a) Barium carbonate is used in list
Add to the ceramics
2. Classify the reactions represented by these equations as an ingredient n
i glazes. Write a chemical equation for
the reaction of barium carbonate with sulfuric acid.
either
Publication single or double2011
date displacement:
Topics (a) Hl(aq) + AgN0 3(aq) ->Agl(s)
nelson, + HN0 311,
chemistry, (aq)chem11, science, (b) Hydrofluoric acid,122,530
HF(aq), is usedViews
to etch glass, which
(b) Fe(s) + CuS0 4(aq)textbookFeS0 4(aq) + Cu(s) is mostly silicon dioxide, Si0 2. The products of this
reaction are water46andFavorites
silicon tetrafluoride.
(c) ZnS(s) + 2 HCI(aq)opensource
Collection -> ZnCI 2(aq) + H2S(g)
Language (d) Cl 2(g) + 2 N H 4Br(aq) -> Br z(l) + 2 N
English H 4CI(aq) (c) Most of the nickel ions n
i a nickel(ll) nitrate solution can be
1 Review
precipitated by adding a solution of potassium carbonate.
3. Write the chemical formula for each of the following
This is the full textbook PDF for Grade 11 Chemistry. More info on it can be found (d) onSulfuric acid removed from used car batteries is neutralized
compounds. Predict the solubility of these compounds n i with sodium carbonate (soda ash).
the publisher's
water:webpage.
(e) Dropping hydrochloric acid on solid zinc sulfide
(a) lead sulfate (in car batteries)
This textbook is legally available to students taking this course at a school that has produces a toxic DOWNLOAD OPTIONS
gas with the odour of rotten eggs.
(b) ammonium phosphate (fertilizer)
paid for the textbook.
(c) calcium sulfate (a component of drywall) 7. Silver compounds are ABBYY expensive.GZMetal manufacturers therefore 2 files
(d) textbook
aluminumis sulfate (used want to recover any waste silver ions from solution. One way
The full size 155M, but I'veinalso
water purification)
made a compressed one that's 80M. No DAISY 2 files
(e) calcium phosphate (i
n bones)
text is changed, the images are just lower quality.
to do this is to precipitate the silver as a compound and then
For users with print-disabilities
(f) barium sulfate (used during stomach X-rays) separate the compound from the mixture using filtration. Use
(g) ammonium carbonate (smelling 22:11:00
salts) EPUB
the solubility table to determine a way of precipitating only2 files
Addeddate 2019-12-10
silver ions from a mixture of dissolved metal ions.
Identifier(h) calcium carbonatechem11(in shells) FULL TEXT 2 files
Identifier-ark
4. Complete the chemical ark:/13960/t7nq06c9m
equations of the following reactions. 8. Research why soaking in vinegar is a convenient method of
ITEM TILE 1 file
Ocr ABBYY
Indicate the state of each FineReader 11.0 (Extended OCR)
compound. removing limescale from the heating coils of a kettle. Write
Ppi 600 -> and classify the chemical PDF reactions involved. # 2 files
(a) ZnCI 2(aq) + KOH(aq)
Scanner(b) Ni(N0 3)2(aq) + N Internet
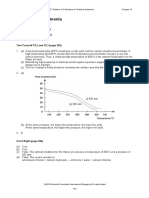
a2C0 3(aq) Archive
-» HTML5 Uploader 1.6.4 9. “Black smokers” emerge SINGLE fromPAGE
cracks n
i the ocean floor2 files
PROCESSED
Year (c) Ba(0H) 2(aq) + K2 2011
S0 4(aq) -» (Figure 9). These black JP2plumes
ZIP are created by superheated
(d) FeS0 4(aq) + K3P0 4(aq) -» water passing through rock. Research the chemical
TORRENT 1 file
(e) ZnS(s) + 2 HCI(aq) -» reactions that cause these plumes to be black. Summarize
(f) CaC0 3(s) + 2 HN0 3(aq) -> your findings in a paragraph. $ tag b t
' Reviews
(g) MgS0 3(aq) + HCI(aq) % Add Review SHOW ALL 22 Files
5. Will
Reviewer: a precipitate
Jack Gordon531form when the
- ★★★★★ following
- March solutions are
8, 2024 8 Original
Subject: Thank
combined?
you If you predict that a precipitate will form, write
Dearest makeworld,
a balanced chemical equation for the reaction. Include
states. If you predict that no reaction occurs, write no
You savedreaction.
my life and I am forever in your debt. I love chemistry and you. Thank you IN COLLECTIONS
ever so much for your
(a) AgN0 3 + addition
K2S0 4 to archive.org.
(d) BaCI 2 + Ca(0H) 2
(b) NH 4CI + Na 2S (e) CuS0 4 + K2C0 3
(c) Pb(N0 3) 2 + l\la3P0 4 Community Texts
Figure 9 An ocean phenomenon
6. Write a balanced chemical equation (with state symbols) for
each of the following double displacement reactions: 8 M B > G O T O NELSON SCIENCE
Community Collections
NEL 4.6 Double Displacement Reactions 177
Uploaded by
makeworld
on December 10, 2019
(183 of 704)
Terms of Service (last updated 12/31/2014)
You might also like
- Training On Liquid Detergents, EnglishDocument71 pagesTraining On Liquid Detergents, EnglishBezakulu MinwouyeletNo ratings yet
- Unit 2 Acids Bases Salts Past QuestionsDocument49 pagesUnit 2 Acids Bases Salts Past QuestionsDwiyasa Irin100% (2)
- Science Class 10 Complete BooksDocument76 pagesScience Class 10 Complete BooksTemsuyanger JamirNo ratings yet
- Aqa Chemistry Using Resources Knowit Gcse H v1Document86 pagesAqa Chemistry Using Resources Knowit Gcse H v1Hossam ElorabyNo ratings yet
- The Chemistry of PhotographyDocument416 pagesThe Chemistry of PhotographyBoris Yershov100% (5)
- Tips For Chemistry ATPDocument4 pagesTips For Chemistry ATPŁílać Mónşťeŕ SublininalsNo ratings yet
- Scientific Trivia (Chemical Engineering)Document4 pagesScientific Trivia (Chemical Engineering)xxkooonxx100% (4)
- Chem2 Laboratory Manual MLS LA1 7 PrelimDocument52 pagesChem2 Laboratory Manual MLS LA1 7 Prelimsampong mga dalereNo ratings yet
- Ammonia: Test Yourself 19.1 (Page 381)Document4 pagesAmmonia: Test Yourself 19.1 (Page 381)Jack Kowman100% (3)
- Acid-Resistant Polydimethylsiloxane Additive For Geothermal Well Cement in 1508C H SO SolutionDocument10 pagesAcid-Resistant Polydimethylsiloxane Additive For Geothermal Well Cement in 1508C H SO SolutionAndhy Arya EkaputraNo ratings yet
- 1 s2.0 S0892687520304842 MainDocument12 pages1 s2.0 S0892687520304842 Mainchaitanya200039No ratings yet
- Anthony M. Wachinski - Environmental Ion Exchange - Principles and Design-Taylor & Francis, Chapman and Hall - CRC (2016) (1) (106-135)Document30 pagesAnthony M. Wachinski - Environmental Ion Exchange - Principles and Design-Taylor & Francis, Chapman and Hall - CRC (2016) (1) (106-135)HARDY EDDISONNo ratings yet
- Avm Chemistry PrelimsDocument6 pagesAvm Chemistry PrelimsTanvi SoniNo ratings yet
- NCERT CHEM01 Q:AsDocument5 pagesNCERT CHEM01 Q:Asvivekabala13No ratings yet
- Chapter 11 Structured QuestionsDocument10 pagesChapter 11 Structured Questionsteresa tsoiNo ratings yet
- Preparingasolublesalt Followupworksheet 746621Document6 pagesPreparingasolublesalt Followupworksheet 746621Quratulain Altaf HusainNo ratings yet
- The Tine Giren at The Head of This Paper Is The Time Allotted For Writing The AnswersDocument8 pagesThe Tine Giren at The Head of This Paper Is The Time Allotted For Writing The Answersannettedenny4No ratings yet
- CH 1 Chemical Reactions and Equations ScienceDocument7 pagesCH 1 Chemical Reactions and Equations ScienceImtiazAhmedNo ratings yet
- NAME: Kaixin Jervey A. Ventura SECTION: 7-Sapphire: WORKSHEET in Science 7Document7 pagesNAME: Kaixin Jervey A. Ventura SECTION: 7-Sapphire: WORKSHEET in Science 7Meynard Garcia CastroNo ratings yet
- Answer Scheme Unit 7Document3 pagesAnswer Scheme Unit 7Jaahnavi KumaresanNo ratings yet
- Chemical Equations 1Document7 pagesChemical Equations 1जggerNaut ClassesNo ratings yet
- Chapter 18 Structured questions 2 - 複本Document18 pagesChapter 18 Structured questions 2 - 複本connieNo ratings yet
- CLS FDN-19!20!10 Che Module-1 Level-1 Chapter-2Document5 pagesCLS FDN-19!20!10 Che Module-1 Level-1 Chapter-2Utkarshini SrivastavaNo ratings yet
- Chemistry: Atoms of Helium in Gaseous State at High TemperatureDocument1 pageChemistry: Atoms of Helium in Gaseous State at High TemperatureQaisar RiazNo ratings yet
- Hydrogen 1Document2 pagesHydrogen 1AarushNo ratings yet
- Mobility of Included Soda in SodaliteDocument4 pagesMobility of Included Soda in SodaliteRogerio CannoniNo ratings yet
- Igcse Chem Acids Bases SaltsDocument3 pagesIgcse Chem Acids Bases SaltsAjay LakshmananNo ratings yet
- Alkene and Alkyne - by Resonance PDFDocument45 pagesAlkene and Alkyne - by Resonance PDFPrasad Yarra100% (1)
- Adobe Scan 05 Feb 2024Document1 pageAdobe Scan 05 Feb 2024krishrajput88888888No ratings yet
- Section:A: L O H S Pbso E Aq H Aq So S PboDocument11 pagesSection:A: L O H S Pbso E Aq H Aq So S PboSrishti TiwariNo ratings yet
- Total Plant Monitoring For An Integrated Steel PlantfinDocument8 pagesTotal Plant Monitoring For An Integrated Steel PlantfinmrtrixsNo ratings yet
- Adobe Scan Aug 17, 2023Document3 pagesAdobe Scan Aug 17, 2023gulatisrishti15No ratings yet
- Group 2 and Group 7Document30 pagesGroup 2 and Group 7lianchen251110No ratings yet
- Actual Repeat Paper 2013Document10 pagesActual Repeat Paper 2013Jasmeet Kaur SandhuNo ratings yet
- NSS Chemistry Part 3 Metals - LQDocument25 pagesNSS Chemistry Part 3 Metals - LQミーチェルNo ratings yet
- L O H S Pbso E Aq H Aq So S Pbo: Section:ADocument10 pagesL O H S Pbso E Aq H Aq So S Pbo: Section:AKalpit SharmaNo ratings yet
- Past Paper - Acids and Alkalis - LQDocument10 pagesPast Paper - Acids and Alkalis - LQapi-3739994100% (2)
- Part 3 MetalsDocument8 pagesPart 3 Metals劉曉晴No ratings yet
- Metals 2020, 10, 1384 5 of 29Document1 pageMetals 2020, 10, 1384 5 of 29P DNo ratings yet
- 4.1 Precipitation and Ionic EquationsDocument14 pages4.1 Precipitation and Ionic EquationstangwindsonNo ratings yet
- CLASS X CHEMISTRY Solution-989556Document7 pagesCLASS X CHEMISTRY Solution-989556abiniveshofficial4708No ratings yet
- 1.chemical Reactions & Equations - CBSE PYQDocument8 pages1.chemical Reactions & Equations - CBSE PYQdeepthividhya0129No ratings yet
- Science PYQDocument82 pagesScience PYQgovidaswamygNo ratings yet
- Analcime Na (Alsi O) H O: CubicDocument3 pagesAnalcime Na (Alsi O) H O: CubicDavid MozoNo ratings yet
- Important Inorganic Trends 4 April FinalDocument62 pagesImportant Inorganic Trends 4 April Finalruchikumari76543No ratings yet
- Chemistry of Representative Elements Lakshya RevisionDocument2 pagesChemistry of Representative Elements Lakshya RevisionRanjan ShuklaNo ratings yet
- 10 Acyl Chlorides and Acids NotesDocument18 pages10 Acyl Chlorides and Acids Noteswith love, alisha.No ratings yet
- (Test 1) NSEC Paper 2015Document18 pages(Test 1) NSEC Paper 2015Devanshu ShahNo ratings yet
- 23-24 X Chem GregoriosDocument10 pages23-24 X Chem Gregoriosarnvt2601No ratings yet
- F3 Chemistry Final Revision - 1617Document6 pagesF3 Chemistry Final Revision - 1617jonas hoNo ratings yet
- LG Bauxite - Alumina RecoveryDocument12 pagesLG Bauxite - Alumina RecoveryKristanto WahyudiNo ratings yet
- Class X NCERT Solutions Chemistry by NTSE GuruDocument5 pagesClass X NCERT Solutions Chemistry by NTSE GuruNTSE GuruNo ratings yet
- CHAP 1.pmd4Document3 pagesCHAP 1.pmd4Ezhil CNo ratings yet
- 83e A Version ChemistryDocument7 pages83e A Version ChemistryVedavathiNo ratings yet
- Aqua RegiaDocument6 pagesAqua RegiaGanesh DixitNo ratings yet
- NSS Chemistry Part 2 Microscopic World I - LQDocument11 pagesNSS Chemistry Part 2 Microscopic World I - LQ[4P29] 王藝樺 WONG NGAI WANo ratings yet
- General Chemistry The Essential Concepts 7th Edition Chang Solutions ManualDocument23 pagesGeneral Chemistry The Essential Concepts 7th Edition Chang Solutions Manualaffableamassor7h7100% (17)
- A Level Chemistry Paper 2 Exam 30Document6 pagesA Level Chemistry Paper 2 Exam 30Anthony AndyNo ratings yet
- S4 HW Ans Sheet (CH - 18 Salts and Neutralization) - SDocument3 pagesS4 HW Ans Sheet (CH - 18 Salts and Neutralization) - STSZ HIN CHANNo ratings yet
- Objective: Alpha Academy ChemistryDocument1 pageObjective: Alpha Academy Chemistrymuhammad AsimNo ratings yet
- CHM1 Enthalpy Change QDocument121 pagesCHM1 Enthalpy Change Qpaolo maldini0% (1)
- Kiangsu-Chekiang College (Shatin) MID-YEAR EXAMINATION (2013-2014) Form 5 Chemistry Marking Scheme: Section A: (30%)Document5 pagesKiangsu-Chekiang College (Shatin) MID-YEAR EXAMINATION (2013-2014) Form 5 Chemistry Marking Scheme: Section A: (30%)wslNo ratings yet
- The P-Block Elements (Boron and Carbon Family) 3.: (Odisha JEE 2004)Document20 pagesThe P-Block Elements (Boron and Carbon Family) 3.: (Odisha JEE 2004)RAGE pubgNo ratings yet
- Chem Question BankDocument71 pagesChem Question BankSai SriramNo ratings yet
- Annual Reports in Organic Synthesis–1982: Annual Reports in Organic SynthesisFrom EverandAnnual Reports in Organic Synthesis–1982: Annual Reports in Organic SynthesisL. G. WadeRating: 5 out of 5 stars5/5 (1)
- Organo AntimonyDocument7 pagesOrgano AntimonyDharmendra Kumar SrivastavaNo ratings yet
- Chem ExamDocument25 pagesChem ExamShela PotoNo ratings yet
- Chem Lab 2Document5 pagesChem Lab 2Amphotorite QuynhNo ratings yet
- Water Resources and Quality: Hazleena Binti Abd HalimDocument18 pagesWater Resources and Quality: Hazleena Binti Abd HalimXendra AqeylaaNo ratings yet
- Molecules 23 00511 v2Document38 pagesMolecules 23 00511 v2Amierson TilendoNo ratings yet
- Home Care Product Brochure 2Document19 pagesHome Care Product Brochure 2Filipe MartinsNo ratings yet
- Corrosion AstroCosmosDocument11 pagesCorrosion AstroCosmosNattapong PongbootNo ratings yet
- Acid-Base ChemistryDocument12 pagesAcid-Base ChemistryNOBLEMANNo ratings yet
- IGCSE Chemistry DefinitionsDocument5 pagesIGCSE Chemistry DefinitionsTanmay Karur100% (1)
- Exp 6Document4 pagesExp 6Rajesh SinghNo ratings yet
- ACIDS and BASES Notes & WorksheetDocument9 pagesACIDS and BASES Notes & WorksheetAdeenaNo ratings yet
- Viva Questions ChemistryDocument9 pagesViva Questions ChemistryAaditya MathurNo ratings yet
- Silo - Tips 2 Write The Chemical Formulas of The Products and Balance The Following Spontaneous ReactionsDocument40 pagesSilo - Tips 2 Write The Chemical Formulas of The Products and Balance The Following Spontaneous ReactionsAkash BhoiNo ratings yet
- Pixl Knowit!: Gcse ChemistryDocument80 pagesPixl Knowit!: Gcse ChemistryBenjamin WatsonNo ratings yet
- Chapter 17Document40 pagesChapter 17khue huynhNo ratings yet
- Defining Ion Exchange CapacityDocument3 pagesDefining Ion Exchange CapacityJoselito CortesNo ratings yet
- Avista TB Scale Inhibitors RO NFDocument4 pagesAvista TB Scale Inhibitors RO NFinejattNo ratings yet
- CBSE Salt AnalysisDocument4 pagesCBSE Salt Analysiskarmanya67% (6)
- US10590362Document14 pagesUS10590362hugo vignoloNo ratings yet
- Getting Started With Chemistry (B) A2 Individual InvestigationDocument42 pagesGetting Started With Chemistry (B) A2 Individual InvestigationBigEvil123No ratings yet
- ExerciseDocument13 pagesExercised anjilappaNo ratings yet
- Science ProjectDocument7 pagesScience ProjectKenzy talkstoomuchNo ratings yet
- Spring 2022 CHEM 123 Recitation Activity #8 - KEYDocument5 pagesSpring 2022 CHEM 123 Recitation Activity #8 - KEYdkNo ratings yet
- Research Article of Precipitation Titration and Its Application by Imroatuz Zakiyah (103194005)Document11 pagesResearch Article of Precipitation Titration and Its Application by Imroatuz Zakiyah (103194005)Zaki Zakiyah100% (3)