Professional Documents
Culture Documents
144.full Cmaj
Uploaded by
Alvin PratamaOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
144.full Cmaj
Uploaded by
Alvin PratamaCopyright:
Available Formats
A N A LY S I S
SCIENCE AND MEDICINE
Immunotherapy made more accessible?
onoclonal antibodies have been developed for the treatment of numerous diseases, but their manufacture is lengthy and labour intensive. A recent discovery may lead to improved production, however. In a new study, Fang and colleagues demonstrate that it is possible to transfer genes that encode for monoclonal antibody into an animal, allowing the expression of a biologically active monoclonal antibody in vivo.1 Antibodies are manufactured for a variety of uses. Immunosuppressive monoclonal antibodies have been created to prevent rejection of transplanted organs. Daclizumab, for example, binds to an interleukin receptor on T-cells, thus preventing lymphocyte activation. Other antibodies target infectious agents to inhibit their replication: palivizumab has been shown to decrease hospital admission rates among children with respiratory syncitial virus infection. The high cost of treatment, however, has limited its use to children with comorbidities and a high risk of complications. An alternative strategy to manufacturing monoclonal antibodies is inducing the body to produce the antibodies itself.2 This has proved difficult, since antibody genes are typically too large to be transported in vectors that would facilitate their easy expression (usually viruses). To date, many attempts to achieve therapeutic serum levels of monoclonal antibodies have failed.1 Fang and colleagues recently developed a method to repackage the DNA of a gene encod-
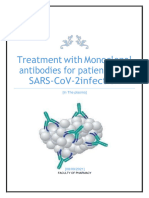
This representation of a model of a human immunoglobulin IgG1 antibody shows the 2 heavy chains in red and the 2 light chains in yellow. Reproduced with the permission of Dr. Mike Clark, Cambridge University.
ing a monoclonal antibody with known antiangiogenic effects in mouse tumour models. The sequences that code for the 2 portions of the antibody known as heavy and light chains were inserted into a viral vector beside a sequence that codes for a peptide derived from the footand-mouth disease virus. The peptide sequence acted as a bridge, enabling the sequences for the heavy and light chains to be read together. With this technique, antibody production was induced in mice with no apparent deleterious effects. Furthermore, after cancer cells were introduced into these mice, the researchers determined that the antibody was indeed biologically active since the mice lived longer and exhib-
ited longer tumour dormancy than control mice. New and improved delivery systems are an important focus of research, given the therapeutic promise of monoclonal antibodies. Although inducing the body to produce antibodies will require ways to control antibody levels and autoimmune reaction,2 the delivery system discovered by Fang and colleagues is a step in the right direction. David Secko, Vancouver
References
1. Fang J, Qian JJ, Yi S, Harding TC, Tu GH, VanRoey M, et al. Stable antibody expression at therapeutic levels using the 2A peptide. Nat Biotech 2005;23(5):584-90. Bakker JM, Bleeker WK, Parren PW. Therapeutic antibody gene transfer: an active approach to passive immunity. Mol Ther 2004;10(3):411-6.
DOI:10.1503/cmaj.050666
2.
144
JAMC 19 JUILL. 2005; 173 (2)
2005 CMA Media Inc. or its licensors
You might also like
- Management of Infections in the Immunocompromised HostFrom EverandManagement of Infections in the Immunocompromised HostBrahm H. SegalNo ratings yet
- DNA Vaccines: Ready For Prime Time?: Michele A. Kutzler and David B. WeinerDocument13 pagesDNA Vaccines: Ready For Prime Time?: Michele A. Kutzler and David B. WeinerantNo ratings yet
- Research Papers On KlebsiellaDocument8 pagesResearch Papers On Klebsiellagzrvpcvnd100% (1)
- Artigo ImunologiaDocument15 pagesArtigo ImunologiaHugo RodriguesNo ratings yet
- 1 s2.0 S0065266005540112 MainDocument33 pages1 s2.0 S0065266005540112 MainComodin PiterNo ratings yet
- ContentServer AspDocument2 pagesContentServer AspDarian EnmanuelNo ratings yet
- Inflammatory Monocytes Suppression of Vaccine Immunity By: References Cites 51 ArticlesDocument11 pagesInflammatory Monocytes Suppression of Vaccine Immunity By: References Cites 51 Articlesthh uuNo ratings yet
- Clin Infect Dis.-2011 - 296-302Document7 pagesClin Infect Dis.-2011 - 296-302Andrezza Furquim Da CruzNo ratings yet
- Nanoparticle Adjuvant Sensing by Tlr7 Enhances Cd8 T Cell Mediated Protection From Listeria Monocytogenes InfectionDocument9 pagesNanoparticle Adjuvant Sensing by Tlr7 Enhances Cd8 T Cell Mediated Protection From Listeria Monocytogenes InfectionEverton MonteiroNo ratings yet
- ReviewDocument35 pagesReviewJaganath RaviNo ratings yet
- covid-finalDocument16 pagescovid-finaldr Amr Aly ReshaNo ratings yet
- Arthropod Vector: Controller of Disease Transmission, Volume 1: Vector Microbiome and Innate Immunity of ArthropodsFrom EverandArthropod Vector: Controller of Disease Transmission, Volume 1: Vector Microbiome and Innate Immunity of ArthropodsStephen K. WikelNo ratings yet
- COVIDVACDocument4 pagesCOVIDVACAdebayo LamikanraNo ratings yet
- Dna VaccineDocument83 pagesDna Vaccineapi-675909478No ratings yet
- Serological Evidence of Merkel Cell Polyomavirus Primary Infections in ChildhoodDocument5 pagesSerological Evidence of Merkel Cell Polyomavirus Primary Infections in ChildhoodzahidNo ratings yet
- To Neutralize or Not, A Key HIV Vaccine Question: Between Bedside and BenchDocument3 pagesTo Neutralize or Not, A Key HIV Vaccine Question: Between Bedside and Benchzee4600No ratings yet
- Journal of Advanced ResearchDocument12 pagesJournal of Advanced ResearchIndriani BaharuddinNo ratings yet
- Biological DNA Sensor: The Impact of Nucleic Acids on Diseases and VaccinologyFrom EverandBiological DNA Sensor: The Impact of Nucleic Acids on Diseases and VaccinologyKen IshiiNo ratings yet
- DNA Vaccines 2005Document7 pagesDNA Vaccines 2005Efrén ChávezNo ratings yet
- Jurnal Vaksin BCG 2Document9 pagesJurnal Vaksin BCG 2niken ambarNo ratings yet
- Vaccine Tcell FluDocument7 pagesVaccine Tcell Fluortizalvarez_arturoNo ratings yet
- Klebsiella Pneumoniae ThesisDocument6 pagesKlebsiella Pneumoniae Thesisaprillaceyjackson100% (1)
- Good Cop, Bad Cop? Interrogating The Immune Responses To Primate Lentiviral VaccinesDocument10 pagesGood Cop, Bad Cop? Interrogating The Immune Responses To Primate Lentiviral VaccinesMuhafizNo ratings yet
- The Final Data CollectionDocument10 pagesThe Final Data Collectionapi-411239539No ratings yet
- Bastian AcuñaDocument11 pagesBastian AcuñaBastiankjj AcuñaNo ratings yet
- Working Paper 3 h5n1Document7 pagesWorking Paper 3 h5n1vijayNo ratings yet
- Vaccine finalDocument34 pagesVaccine finalfmznn588kcNo ratings yet
- Vaccines of The Future Nossal2011Document5 pagesVaccines of The Future Nossal2011Dr Varshil ShahNo ratings yet
- Immunotherapeutic Strategies for the Treatment of GliomaFrom EverandImmunotherapeutic Strategies for the Treatment of GliomaNo ratings yet
- 140794.3 20210324105625 CoveredDocument16 pages140794.3 20210324105625 CoveredBruno RamírezNo ratings yet
- Covid 19 Vaccines An Australian ReviewDocument18 pagesCovid 19 Vaccines An Australian ReviewLMCG DocketNo ratings yet
- New Generation of Vaccines Prepared Using Genetically Modified MicroorganismsDocument7 pagesNew Generation of Vaccines Prepared Using Genetically Modified MicroorganismsSyed Waseem AbbasNo ratings yet
- Monoclonal AntibodyDocument8 pagesMonoclonal AntibodySadaf Sumdani NirvikNo ratings yet
- Future of Medical ImmunologyDocument2 pagesFuture of Medical ImmunologyyassinsaadNo ratings yet
- Hiv VaccineDocument6 pagesHiv VaccineNeo Mervyn MonahengNo ratings yet
- Vaccine HistoryDocument35 pagesVaccine HistoryEhed AymazNo ratings yet
- Review of Flavivirus EditedDocument9 pagesReview of Flavivirus Editedapi-318473384No ratings yet
- Cover StoryDocument3 pagesCover StoryaggarsandeepNo ratings yet
- tmpBF53 TMPDocument6 pagestmpBF53 TMPFrontiersNo ratings yet
- HIV Vaccine Research in Canada: AIDS Research and TherapyDocument3 pagesHIV Vaccine Research in Canada: AIDS Research and Therapyerika quitianNo ratings yet
- Fimmu 10 00594Document13 pagesFimmu 10 00594Aleksandar DimkovskiNo ratings yet
- Identification and Construction of A Multi Epitopes Vaccine Design Against Klebsiella Aerogenes: Molecular Modeling StudyDocument16 pagesIdentification and Construction of A Multi Epitopes Vaccine Design Against Klebsiella Aerogenes: Molecular Modeling StudySamer ShamshadNo ratings yet
- DNA Vaccination: Advanced Microbiology Course Prof: Rula DarwishDocument30 pagesDNA Vaccination: Advanced Microbiology Course Prof: Rula Darwishmalak amerNo ratings yet
- FULLTEXT01Document100 pagesFULLTEXT01sdfijaksdgNo ratings yet
- Increased Humoral Immunity by DNA Vaccination Using An A-Tocopherol-Based AdjuvantDocument8 pagesIncreased Humoral Immunity by DNA Vaccination Using An A-Tocopherol-Based AdjuvantFakultas Kedokteran UnhanNo ratings yet
- Schiller JT - 09Document6 pagesSchiller JT - 09Bruno RalhaNo ratings yet
- COVID-19 Vaccines - Final VersionDocument11 pagesCOVID-19 Vaccines - Final VersionTom BiusoNo ratings yet
- Covid-19 vaccine from Pfizer and BioNTech shows positive resultsDocument7 pagesCovid-19 vaccine from Pfizer and BioNTech shows positive resultsGabriela FernandezNo ratings yet
- Toxicol Pathol 2008 Cotrim 97 103Document8 pagesToxicol Pathol 2008 Cotrim 97 103rinirhynNo ratings yet
- 10.1007/978 3 662 46410 6Document522 pages10.1007/978 3 662 46410 6Francisco Ibañez Irribarra100% (1)
- An Integrated Computational Framework To Design A Multi Epitopes Vaccine Against Mycobacterium TuberculosisDocument18 pagesAn Integrated Computational Framework To Design A Multi Epitopes Vaccine Against Mycobacterium TuberculosisSamer ShamshadNo ratings yet
- TUBE MostCited 1Document6 pagesTUBE MostCited 1jibin2008No ratings yet
- Human Vaccines: Emerging Technologies in Design and DevelopmentFrom EverandHuman Vaccines: Emerging Technologies in Design and DevelopmentKayvon ModjarradNo ratings yet
- Research Papers On Monoclonal AntibodiesDocument8 pagesResearch Papers On Monoclonal Antibodiesfxigfjrhf100% (1)
- Kelainan RefraksiDocument39 pagesKelainan RefraksiAlvin PratamaNo ratings yet
- Case Report Typhoid Fever (A01.0)Document32 pagesCase Report Typhoid Fever (A01.0)Alvin PratamaNo ratings yet
- Typhoid Fever Case ReportDocument32 pagesTyphoid Fever Case ReportAlvin PratamaNo ratings yet
- MR 2 Juni OA, AzotemiaDocument32 pagesMR 2 Juni OA, AzotemiaAlvin PratamaNo ratings yet
- POMRDocument3 pagesPOMRAlvin PratamaNo ratings yet
- BradikardiDocument11 pagesBradikardiAlvin PratamaNo ratings yet
- DT Refractive ErrorsDocument50 pagesDT Refractive ErrorsAlvin PratamaNo ratings yet
- Tia Abcd2 Tool 1Document2 pagesTia Abcd2 Tool 1Siti RahmahNo ratings yet
- Amblyopia: Alvin Pratama Jauharie I11111063Document27 pagesAmblyopia: Alvin Pratama Jauharie I11111063Alvin PratamaNo ratings yet
- Voucher Printout Weis Brave Frontier PDFDocument1 pageVoucher Printout Weis Brave Frontier PDFAlvin PratamaNo ratings yet
- Abdominal ExaminationDocument19 pagesAbdominal ExaminationAlvin PratamaNo ratings yet
- BradikardiDocument11 pagesBradikardiAlvin PratamaNo ratings yet
- POMR 5. TyphoidDocument3 pagesPOMR 5. TyphoidAlvin PratamaNo ratings yet
- POMR 5. TyphoidDocument3 pagesPOMR 5. TyphoidAlvin PratamaNo ratings yet
- Tabel Dan Gambar Perkembangan Embrio ManusiaDocument11 pagesTabel Dan Gambar Perkembangan Embrio ManusiaAlvin PratamaNo ratings yet
- UrinaryRetention 508Document16 pagesUrinaryRetention 508Faiz NazriNo ratings yet
- Kalender 2015 Indonesia - Design - 05 - Art RightDocument1 pageKalender 2015 Indonesia - Design - 05 - Art RightMyc-lNo ratings yet
- Guillain Barre SyndromeDocument13 pagesGuillain Barre SyndromeAlvin PratamaNo ratings yet
- Acute Lymphoblastic Leukemia: Review ArticleDocument14 pagesAcute Lymphoblastic Leukemia: Review ArticleAlvin PratamaNo ratings yet
- Pediatric Acute Lymphoblastic LeukemiaDocument9 pagesPediatric Acute Lymphoblastic LeukemiaAlvin PratamaNo ratings yet
- Immune Thrombocytopenic Purpura PDFDocument12 pagesImmune Thrombocytopenic Purpura PDFAlvin PratamaNo ratings yet
- Plasmodium Knowlesi: Human Infection Detected by Rapid Diagnostic Tests For MalariaDocument3 pagesPlasmodium Knowlesi: Human Infection Detected by Rapid Diagnostic Tests For MalariaAlvin PratamaNo ratings yet
- Viewarticle 730670 PrintDocument6 pagesViewarticle 730670 PrintAlvin PratamaNo ratings yet
- Viewarticle 752509 PrintDocument12 pagesViewarticle 752509 PrintAlvin PratamaNo ratings yet
- TABLE 8-3 Cough and Hemoptysis Problem and Sputum Associated Symptoms and Setting Acute InflammationDocument2 pagesTABLE 8-3 Cough and Hemoptysis Problem and Sputum Associated Symptoms and Setting Acute InflammationAlvin PratamaNo ratings yet
- Hyperreflexia in GBSDocument5 pagesHyperreflexia in GBSAlvin PratamaNo ratings yet
- Penuntun Praktikum Modul KV Kurfak 05.08Document29 pagesPenuntun Praktikum Modul KV Kurfak 05.08Alvin PratamaNo ratings yet
- Tumour Necrosis Factor-Inhibitors and The Reactivation of Latent Tuberculosis InfectionDocument4 pagesTumour Necrosis Factor-Inhibitors and The Reactivation of Latent Tuberculosis InfectiontcisiliaNo ratings yet
- JO1 Plar GIE and MIEDocument2 pagesJO1 Plar GIE and MIEKaloy PlarNo ratings yet
- Scada/Ems/Dms: Electric Utilities Networks & MarketsDocument12 pagesScada/Ems/Dms: Electric Utilities Networks & MarketsdoquocdangNo ratings yet
- Gothic Novel Definition: of The Gothic Initiated by WalpoleDocument5 pagesGothic Novel Definition: of The Gothic Initiated by Walpolesehrish noreenNo ratings yet
- Career Change TheoryDocument29 pagesCareer Change TheoryPamela Joy SeriosaNo ratings yet
- Social Skill Training To Improve Social Interactions in SkizofreniaDocument8 pagesSocial Skill Training To Improve Social Interactions in SkizofreniaFebby Rosa AnnisafitrieNo ratings yet
- The Lack of Sports Facilities Leads To Unhealthy LIfestyle Among StudentsReportDocument22 pagesThe Lack of Sports Facilities Leads To Unhealthy LIfestyle Among StudentsReportans100% (3)
- Rufino Luna, Et - Al V CA, Et - Al (G.R. No. 100374-75)Document3 pagesRufino Luna, Et - Al V CA, Et - Al (G.R. No. 100374-75)Alainah ChuaNo ratings yet
- Meaning of Population EducationDocument28 pagesMeaning of Population Educationanon_896879459No ratings yet
- Controllogix Enhanced Redundancy System: User ManualDocument254 pagesControllogix Enhanced Redundancy System: User ManualCristian RomeroNo ratings yet
- Borang Pemarkahan RIMUP (Dazanak Tavantang)Document11 pagesBorang Pemarkahan RIMUP (Dazanak Tavantang)ELLYE ORNELLA SULIMAN MoeNo ratings yet
- Review On Internal Combustion Engine Vibrations and MountingsDocument12 pagesReview On Internal Combustion Engine Vibrations and MountingsSanthosh KumarNo ratings yet
- ENGLSIH LISTENING SKILLS (Question Paper) PDFDocument2 pagesENGLSIH LISTENING SKILLS (Question Paper) PDFGagan H RNo ratings yet
- HL-Series Service Manual 96-8710 English June 1998Document234 pagesHL-Series Service Manual 96-8710 English June 1998kumbrov100% (1)
- 2012 09 16 Our Anchor in Times of StormDocument3 pages2012 09 16 Our Anchor in Times of StormJohn S. KodiyilNo ratings yet
- The Flower of Services A Study On Private Universities in Sylhet CityDocument11 pagesThe Flower of Services A Study On Private Universities in Sylhet CityLatif AbdulNo ratings yet
- IELTS Reading Practice Test 11: Waking Numbness or Sleep ParalysisDocument3 pagesIELTS Reading Practice Test 11: Waking Numbness or Sleep ParalysisPiyush ChaudharyNo ratings yet
- 5th Grade 13-14 Math Common Core Standards by QuarterDocument3 pages5th Grade 13-14 Math Common Core Standards by QuartermrkballNo ratings yet
- Behavioral Finance-An OverviewDocument46 pagesBehavioral Finance-An OverviewriyasacademicNo ratings yet
- Design and Construction of A Wireless We PDFDocument69 pagesDesign and Construction of A Wireless We PDFPeculiar EbhueleNo ratings yet
- Nuisance or Natural and Healthy Should Monthly Menstruation Be Optional For Women PDFDocument3 pagesNuisance or Natural and Healthy Should Monthly Menstruation Be Optional For Women PDFDieWeisseLeserinNo ratings yet
- Regents Prep 4Document7 pagesRegents Prep 4api-24284785450% (2)
- Fee Schedule Yellow (A) UpdatedDocument2 pagesFee Schedule Yellow (A) Updatedade melly septianaNo ratings yet
- Review Article: Neurophysiological Effects of Meditation Based On Evoked and Event Related Potential RecordingsDocument12 pagesReview Article: Neurophysiological Effects of Meditation Based On Evoked and Event Related Potential RecordingsSreeraj Guruvayoor SNo ratings yet
- Xploring FLN: Resource Pack On Foundational Literacy and Numeracy (FLN)Document119 pagesXploring FLN: Resource Pack On Foundational Literacy and Numeracy (FLN)rakeshahlNo ratings yet
- Tort of Negligence Modefied 1Document20 pagesTort of Negligence Modefied 1yulemmoja100% (2)
- Harry Potter Essay 1 OutlineDocument10 pagesHarry Potter Essay 1 OutlinevickaduzerNo ratings yet
- Bloock Diagram Reduction SummaryDocument10 pagesBloock Diagram Reduction SummaryLakshay KhichiNo ratings yet
- We The Digital Citizens - Common Sense EducationDocument5 pagesWe The Digital Citizens - Common Sense EducationMs. Rizza MagnoNo ratings yet
- Two Contributions To The Foundations of Set TheoryDocument9 pagesTwo Contributions To The Foundations of Set TheoryDietethiqueNo ratings yet
- The People of The Materia Medica World: A Comparative Materia Medica Part-1Document5 pagesThe People of The Materia Medica World: A Comparative Materia Medica Part-1Paromita Dasgupta50% (2)