Professional Documents
Culture Documents
Boyles Law
Uploaded by
Iking De Lara0 ratings0% found this document useful (0 votes)
751 views26 pages Here are the step-by-step solutions to the Boyle's Law problems:
1) P1V1 = P2V2
40 mmHg * 12.3 L = 60 mmHg * V2
V2 = 12.3 * 60/40 = 9.225 L
2) P1V1 = P2V2
1 atm * 3.60 L = 2.5 atm * V2
V2 = 3.60 * 1/2.5 = 1.44 L
3) P1V1 = P2V2
1 atm * 400 ft^3 = P2 * 3 ft^3
P2 = 1 * 400/3 = 133.3
Original Description:
Original Title
Boyles-Law.ppt
Copyright
© © All Rights Reserved
Available Formats
PPT, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this Document Here are the step-by-step solutions to the Boyle's Law problems:
1) P1V1 = P2V2
40 mmHg * 12.3 L = 60 mmHg * V2
V2 = 12.3 * 60/40 = 9.225 L
2) P1V1 = P2V2
1 atm * 3.60 L = 2.5 atm * V2
V2 = 3.60 * 1/2.5 = 1.44 L
3) P1V1 = P2V2
1 atm * 400 ft^3 = P2 * 3 ft^3
P2 = 1 * 400/3 = 133.3
Copyright:
© All Rights Reserved
Available Formats
Download as PPT, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
751 views26 pagesBoyles Law
Uploaded by
Iking De Lara Here are the step-by-step solutions to the Boyle's Law problems:
1) P1V1 = P2V2
40 mmHg * 12.3 L = 60 mmHg * V2
V2 = 12.3 * 60/40 = 9.225 L
2) P1V1 = P2V2
1 atm * 3.60 L = 2.5 atm * V2
V2 = 3.60 * 1/2.5 = 1.44 L
3) P1V1 = P2V2
1 atm * 400 ft^3 = P2 * 3 ft^3
P2 = 1 * 400/3 = 133.3
Copyright:
© All Rights Reserved
Available Formats
Download as PPT, PDF, TXT or read online from Scribd
You are on page 1of 26
Boyle’s Law
Discussant: Jeffrey Flores (Sir Jeff )
What is Boyle’s Law?
• Boyle’s Law is one of the laws in physics that
concern the behaviour of gases
• When a gas is under pressure it takes up less
space:
• The higher the pressure, the smaller the
volume
• Boyles Law tells us about the relationship
between the volume of a gas and its pressure
at a constant temperature
• The law states that pressure is inversely
proportional to the volume
How can we write Boyle’s Law as a
formula?
• Pressure is inversely • This is more usually
proportional to the written as:
volume and can be
written as:
• Pressure a 1/volume • Pressure =
constant
P=pressure in N/m2 volume
V=volume in dm3 (litres) • PV=k
k=constant • P1V1=P2V2
How can we investigate Boyle’s Law?
• When investigating Boyles law a given
volume of gas is sucked into a cylinder
and the end is sealed
• The temperature of the gas is kept
constant
• Using several equal weights we can apply
increasing pressure to the gas
• We can calculate the pressure by dividing
the force applied by the area of the top of
the cylinder
• The volume will be shown on the scale on
the cylinder
Boyle’s Law apparatus
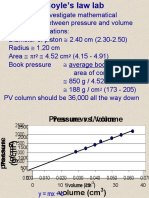
Below are some results of an experiment
Pressure p Volume V PxV
1.1 40 44
1.7 26
2.2 20
2.6 17
• Calculate pV (pressure x volume) for each set of
results. What do you notice?
What these experimental results show
• The pressure x volume for each set of
results remains constant
• This is called Boyle’s Law
• For a fixed mass of gas, at constant
temperature, pV = constant or
P1 x V1 = P2 x V2
• Let us look at the results again
Here are the results of the experiment
Pressure p Volume V PxV
1.1 40 44
1.7 26 44
2.2 20 44
2.6 17 44
• Did you notice that if p is doubled, V is halved?
• If p increases to 3 times as much, V decreases to a 1/3rd .
This means:
• Volume is inversely proportional to pressure, or
V1
p
What sort of graphs would this
data give?
• If we plot volume directly against pressure
we would get a downwards curve showing
that volume gets smaller as the pressure
gets larger, and vice versa.
Another way of plotting the data
• Curved lines are hard to recognise, so
we plot the volume against the
reciprocal of pressure (ie. 1/p)
• This time the points lie close to a
straight line through the origin.
• This means volume is directly
proportional to 1/pressure or
• volume is inversely proportional to
pressure
This leads us back to Boyle’s
Law
Boyle’s Law: for a fixed mass
of gas kept at constant
temperature the volume of
the gas is inversely
proportional to its pressure.
Problem:
• A deep sea diver is
working at a depth
where the pressure is
3.0 atmospheres. He is
breathing out air
bubbles. The volume of
each air bubble is 2
cm2. At the surface the
pressure is 1
atmosphere. What is
the volume of each
bubble when it reaches
the surface?
How we work this out:
• We assume that the temperature is constant, so
Boyle’s Law applies:
• Formula first: P1 x V1 = P2 x V2
• Then numbers:= 1.0 x 2 = 3.0 x V2
• Now rearrange the numbers so that you have V2
on one side, and the rest of the numbers on the
other side of the ‘equals’ symbol.
Here’s what you should have calculated
V2 = 3.0 x 2
1.0
therefore volume of bubbles = 6 cm3
Note that P1 and P2 have the same unit, as will V1 and V2
This powerpoint was kindly donated to
www.worldofteaching.com
http://www.worldofteaching.com is home to over a
thousand powerpoints submitted by teachers. This is a
completely free site and requires no registration. Please
visit and I hope it will help in your teaching.
Problem #1: A gas occupies 12.3 liters at a
pressure of 40.0 mmHg. What is the volume
when the pressure is increased to 60.0
mmHg?
Problem #2: If a gas at 25.0 °C occupies
3.60 liters at a pressure of 1.00 atm, what
will be its volume at a pressure of 2.50 atm
• Problem #3: To what pressure must a gas
be compressed in order to get into a 3.00
cubic foot tank the entire weight of a gas
that occupies 400.0 cu. ft. at standard
pressure?
• Problem #4: A gas occupies 1.56 L at
1.00 atm. What will be the volume of this
gas if the pressure becomes 3.00 atm?
• Problem #5: A gas occupies 11.2 liters at
0.860 atm. What is the pressure if the
volume becomes 15.0 L
• Problem #6: 500.0 mL of a gas is
collected at 745.0 mmHg. What will the
volume be at standard pressure
• Problem #7: Convert 350.0 mL at 740.0
mmHg to its new volume at standard
pressure.
Problem #8: Convert 338 L at 63.0 atm to
its new volume at standard pressure
• Problem #9: Convert 273.15 mL at 166.0
kPa to its new volume at standard
pressure.
(166.0 kPa) (273.15 mL) = (101.325 kPa) (x)
Problem #10: Convert 77.0 L at 18.0 mmHg
to its new volume at standard pressure.
• (18.0 mmHg) (77.0 L) = (760.0 mmHg) (x)
• Problem #11: When the pressure on a
gas increases, will the volume increase or
decrease?
Volume will decrease.
Problem #12: If the pressure on a gas is
decreased by one-half, how large will the
volume change be?
• It will double in size.
• Problem #13: A gas occupies 4.31 liters at a
pressure of 0.755 atm. Determine the volume if
the pressure is increased to 1.25 atm.
(0.755 atm) (4.31 liters) = (1.25 atm) (x)
Problem #14: 600.0 mL of a gas is at a pressure
of 8.00 atm. What is the volume of the gas at 2.00
atm?
(8.00 atm) (600.0 mL) = (2.00 atm) (x)
Problem #15: 400.0 mL of a gas are under a
pressure of 800.0 torr. What would the volume of
the gas be at a pressure of 1000.0 torr?
• (800.0 torr) (400.0 mL) = (1000.0 torr) (x)
You might also like
- Daily Lesson Log Boyle's LawDocument2 pagesDaily Lesson Log Boyle's LawANGELIQUE ANTONIO100% (6)
- Boyle's & Charles' Law WorksheetDocument6 pagesBoyle's & Charles' Law WorksheetMary Grace Jerna Artazo Nozal-CuadraNo ratings yet
- Combined Gas LawDocument3 pagesCombined Gas Lawmarigold suarez0% (1)
- Boyles Law Power PointDocument9 pagesBoyles Law Power Pointapi-19917867100% (1)
- Boyle's Law Activity SheetDocument1 pageBoyle's Law Activity SheetErnesto G. Flores Jr.No ratings yet
- Circus Charlie: Charles' Law!: A Strategic Intervention Material in ChemistryDocument12 pagesCircus Charlie: Charles' Law!: A Strategic Intervention Material in ChemistryDon King Evangelista100% (1)
- 3Q Project Impt Doc Files COMPLETEDocument6 pages3Q Project Impt Doc Files COMPLETEroryianNo ratings yet
- Gay Lussac - S Law WorksheetDocument2 pagesGay Lussac - S Law WorksheetCenando Bodanio100% (3)
- Detailed Lesson Plan in Science 10: Charles' LawDocument6 pagesDetailed Lesson Plan in Science 10: Charles' LawHaji Darell Bagtang95% (43)
- Boyles LawDocument31 pagesBoyles LawJanetMagnayeLapitan100% (1)
- Science 10 q4 Week 1Document11 pagesScience 10 q4 Week 1Jacqueline FabiaNo ratings yet
- Conversion and UnitsDocument6 pagesConversion and UnitsAira VillarinNo ratings yet
- The Temperature-Pressure Relationship Gay-Lussac's LawDocument8 pagesThe Temperature-Pressure Relationship Gay-Lussac's LawElbert NatalNo ratings yet
- Q4-Science-9-Week 4Document4 pagesQ4-Science-9-Week 4jholan debelenNo ratings yet
- Charles's Law DemoDocument20 pagesCharles's Law DemoJackylou SaludesNo ratings yet
- Intro To Gases and Gas LawsDocument45 pagesIntro To Gases and Gas LawsAmeerah Sophia Tanedo100% (2)
- LAS 2: Boyle's Law Pre-TestDocument4 pagesLAS 2: Boyle's Law Pre-TestSalve SerranoNo ratings yet
- Boyles Law EditedDocument10 pagesBoyles Law EditedRico FernandezNo ratings yet
- Squashing The Bottle-Ideal Gas LawDocument1 pageSquashing The Bottle-Ideal Gas LawSarah Candelaria ArcellanaNo ratings yet
- Co-Heat Engine-Grade-9-FinalDocument8 pagesCo-Heat Engine-Grade-9-FinalApolonio Pamittan Jr.No ratings yet
- Summative Test in Grade 10 ScienceDocument2 pagesSummative Test in Grade 10 Sciencecherry salvacionNo ratings yet
- Lesson Plan in Demo TeachingDocument8 pagesLesson Plan in Demo TeachingHanna LamesNo ratings yet
- 2 - Activity Factors Affecting ClimateDocument7 pages2 - Activity Factors Affecting ClimateCes Michaela CadividaNo ratings yet
- Module 3 Impulse Momentum BelloDocument13 pagesModule 3 Impulse Momentum BelloDanny Riel BarachinaNo ratings yet
- Apply Boyle's Law Word ProblemDocument3 pagesApply Boyle's Law Word ProblemAm ThalNo ratings yet
- S10Fe-Iia-B-47: The Learners Shall Be Able ToDocument8 pagesS10Fe-Iia-B-47: The Learners Shall Be Able Toxoxkakidoxox100% (4)
- Department of Education: Learning Activity SheetDocument3 pagesDepartment of Education: Learning Activity SheetKaren May UrlandaNo ratings yet
- Detailed Lesson Plan in Science 10 - Chemistry: Boyle's Law: Volume and Pressure RelationshipDocument22 pagesDetailed Lesson Plan in Science 10 - Chemistry: Boyle's Law: Volume and Pressure RelationshipJehu PabloNo ratings yet
- Avogadros LawDocument5 pagesAvogadros LawAgyao Yam FaithNo ratings yet
- Module 7 Rate of ReactionsDocument38 pagesModule 7 Rate of ReactionsAliyah Khairani100% (1)
- Grade 10first TQDocument3 pagesGrade 10first TQJerbs PacundoNo ratings yet
- General Physics 1 VECTORDocument57 pagesGeneral Physics 1 VECTORPortia Egken100% (1)
- The Philippines Is Prone To Tropical CyclonesDocument4 pagesThe Philippines Is Prone To Tropical CyclonesfarhanieNo ratings yet
- G10 BiomoleculesDocument49 pagesG10 BiomoleculesMc AcebarNo ratings yet
- Activities For Mole ConceptDocument4 pagesActivities For Mole ConceptJunard Asentista100% (1)
- Combined Gas Law (DLP)Document6 pagesCombined Gas Law (DLP)Marvin EusebioNo ratings yet
- I. Objectives: Detailed Science Lesson Plan Grade Level 10 Quarter/Dom AIN 4 Quarter Week & Day NO. Page NoDocument3 pagesI. Objectives: Detailed Science Lesson Plan Grade Level 10 Quarter/Dom AIN 4 Quarter Week & Day NO. Page NoDinz Guanzon TayactacNo ratings yet
- For Combined Gas LawDocument44 pagesFor Combined Gas LawApril Bartolome Flores100% (1)
- Gay-Lussac's Law Problems and SolutionsDocument1 pageGay-Lussac's Law Problems and SolutionsBasic PhysicsNo ratings yet
- LP - Charles LawDocument4 pagesLP - Charles Lawrichele rectoNo ratings yet
- Mass in KG X 9.8 M/s Force /areaDocument2 pagesMass in KG X 9.8 M/s Force /areaLeil RiegoNo ratings yet
- Boyles Law Lesson PlanDocument4 pagesBoyles Law Lesson Planbernadeth barajasNo ratings yet
- Kinetic Molecular TheoryDocument3 pagesKinetic Molecular TheoryGarren Jude Aquino100% (1)
- LAS 4 - Recognize The Major Categories of Biomolecules Such As Carbohydrates, Lipids, Proteins, and Nucleic AcidsDocument3 pagesLAS 4 - Recognize The Major Categories of Biomolecules Such As Carbohydrates, Lipids, Proteins, and Nucleic AcidsSalve SerranoNo ratings yet
- Properties of GasesDocument14 pagesProperties of GasesNeo EpeNo ratings yet
- Charles LawDocument23 pagesCharles LawJanetMagnayeLapitan100% (2)
- Q4-Worksheet-Week 5Document7 pagesQ4-Worksheet-Week 5Gian EvangelistaNo ratings yet
- APPLICATION FORM For PCWHS-STE AdmissionDocument6 pagesAPPLICATION FORM For PCWHS-STE AdmissionSophia Pauline HernandezNo ratings yet
- The Magnetic Property of An Atom and Atoms Atomic OrbitalsDocument12 pagesThe Magnetic Property of An Atom and Atoms Atomic OrbitalsJanne Lorraine Garcia-EleazarNo ratings yet
- Boyles LawDocument17 pagesBoyles LawRuss Afuang100% (1)
- Lesson Plan in Science 10Document12 pagesLesson Plan in Science 10Beryl Andal100% (1)
- Summative Test 4 - PsDocument4 pagesSummative Test 4 - PsKennedy Fieldad VagayNo ratings yet
- Boyle's LawDocument3 pagesBoyle's LawGarren Jude AquinoNo ratings yet
- Detailed Lesson Plan in Grade 10 Combined Gas LawDocument5 pagesDetailed Lesson Plan in Grade 10 Combined Gas LawJoriel Jordan CruzNo ratings yet
- g9dllfeb26ELASTIC COLLISION - DocxfinalDocument16 pagesg9dllfeb26ELASTIC COLLISION - DocxfinalChe MandaneNo ratings yet
- Science: Quarter 4 - Module 6: How Heat Transfer and Energy Transformation Makes Heat Engine WorkDocument11 pagesScience: Quarter 4 - Module 6: How Heat Transfer and Energy Transformation Makes Heat Engine WorkEECezar JeicyyuiNo ratings yet
- EncarguezGweenA. DIAMOND STEM GC11DL-I1-j-48Document14 pagesEncarguezGweenA. DIAMOND STEM GC11DL-I1-j-48Lee Sung YeolNo ratings yet
- L A S - S Ci: Science Activity Sheet Quarter 1-MELC 1 Week 1Document15 pagesL A S - S Ci: Science Activity Sheet Quarter 1-MELC 1 Week 1Benedick Zorilla ZaspaNo ratings yet
- Circular Motion DLP 062019Document5 pagesCircular Motion DLP 062019IRISNo ratings yet
- Department of Education: Learning Activity SheetDocument4 pagesDepartment of Education: Learning Activity SheetKaren May UrlandaNo ratings yet
- Module 1.3: Kinetic Molecular Theory: Lagay National High School Calauag West DistrictDocument2 pagesModule 1.3: Kinetic Molecular Theory: Lagay National High School Calauag West DistrictJAYNAROSE IBAYAN100% (1)
- Boyle's LawDocument15 pagesBoyle's LawMani KandanNo ratings yet
- Science NotebookDocument8 pagesScience NotebookReichstadtNo ratings yet
- Boyles Law Lab AnswersDocument5 pagesBoyles Law Lab AnswersShemelis AragawNo ratings yet
- Gas LawsDocument4 pagesGas LawsDar W. InNo ratings yet
- Chapter 5 GasesDocument37 pagesChapter 5 GasesNeally WeallyNo ratings yet
- Hypothesis, Laws, TheoriesDocument30 pagesHypothesis, Laws, TheoriesCeline PlacidoNo ratings yet
- Science 10 - Week 28Document4 pagesScience 10 - Week 28Mira VeranoNo ratings yet
- Chapter 5 GasesDocument20 pagesChapter 5 GasesKevin MellizaNo ratings yet
- Boyles Law Practice SheetDocument2 pagesBoyles Law Practice SheetJemuel Arias (Jem)No ratings yet
- 6 - ch5 Aa 0Document49 pages6 - ch5 Aa 0Edlyn RamirezNo ratings yet
- Pressure of Gases: General Chemistry 1Document9 pagesPressure of Gases: General Chemistry 1Daniel Corcino50% (2)
- Chapter 1 ThermodynamicsDocument337 pagesChapter 1 ThermodynamicsCandice PeñaNo ratings yet
- BLB - CH10 Ejercicios ResueltosDocument38 pagesBLB - CH10 Ejercicios ResueltosRosa Elsy Puentes LondoñoNo ratings yet
- Experiment Gas LawsDocument8 pagesExperiment Gas Lawsapi-254428474No ratings yet
- KTG Neet PDF Lecture NotesDocument67 pagesKTG Neet PDF Lecture NotesKRISHNANo ratings yet
- Chem Notes Chapter 5 - GasesDocument81 pagesChem Notes Chapter 5 - GasesjohnNo ratings yet
- Direct and Inverse ProportionDocument18 pagesDirect and Inverse ProportionJanvierNo ratings yet
- Compressibility of NGDocument15 pagesCompressibility of NGmark_fish22No ratings yet
- VIII GASEOU STATE - I FC Setting Completed Niharika Mam Final 220-266Document51 pagesVIII GASEOU STATE - I FC Setting Completed Niharika Mam Final 220-266Augustine Joe JNo ratings yet
- Relationship BTWN Pressure & VolumeDocument1 pageRelationship BTWN Pressure & Volumeareyouthere92No ratings yet
- Gfe - Week 2 - Part 1 - 2018-05-28Document43 pagesGfe - Week 2 - Part 1 - 2018-05-28AdeelAbbasNo ratings yet
- Relating Pressure Volume Amount and Temperature The Ideal Gas Law 4Document19 pagesRelating Pressure Volume Amount and Temperature The Ideal Gas Law 4tina pixieNo ratings yet
- Characteristics Compressed Air: ThermodynamicsDocument9 pagesCharacteristics Compressed Air: ThermodynamicsAlex UliniciNo ratings yet
- CHE486 Lab 6 Property Measurement To Send WsDocument23 pagesCHE486 Lab 6 Property Measurement To Send WsRazali RamlanNo ratings yet
- G10 SCI Q4WK1 LAS1-checked-and-editedDocument5 pagesG10 SCI Q4WK1 LAS1-checked-and-editedKirsten Tiffany TriasNo ratings yet
- Chem M9 Gas LawsDocument25 pagesChem M9 Gas LawsMa Perpetua Bardelas BaldescoNo ratings yet
- 3-2 Boyles Law SolDocument4 pages3-2 Boyles Law SolyNo ratings yet
- 4q Science 10 PTDocument2 pages4q Science 10 PTBecky ArmstrongNo ratings yet
- Gas LawsDocument80 pagesGas LawsChennille Ann Bleu GundayaoNo ratings yet