Professional Documents
Culture Documents
4.5 Oxymercuration
Uploaded by
Dawit Birhanu0 ratings0% found this document useful (0 votes)
57 views6 pagesOxymercuration-demercuration is a reaction where an alkene is treated with mercuric acetate and an alcohol to form an ether. Mercuration involves the electrophilic addition of a mercuric salt to the alkene, forming an alkyl-mercury derivative. The mercury is then removed in a subsequent demercuration step to yield the ether product. The overall reaction follows Markovnikov regioselectivity and is anti-stereospecific, proceeding by a mechanism similar to other electrophilic addition reactions.
Original Description:
Copyright
© © All Rights Reserved
Available Formats
PPTX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentOxymercuration-demercuration is a reaction where an alkene is treated with mercuric acetate and an alcohol to form an ether. Mercuration involves the electrophilic addition of a mercuric salt to the alkene, forming an alkyl-mercury derivative. The mercury is then removed in a subsequent demercuration step to yield the ether product. The overall reaction follows Markovnikov regioselectivity and is anti-stereospecific, proceeding by a mechanism similar to other electrophilic addition reactions.
Copyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
57 views6 pages4.5 Oxymercuration
Uploaded by
Dawit BirhanuOxymercuration-demercuration is a reaction where an alkene is treated with mercuric acetate and an alcohol to form an ether. Mercuration involves the electrophilic addition of a mercuric salt to the alkene, forming an alkyl-mercury derivative. The mercury is then removed in a subsequent demercuration step to yield the ether product. The overall reaction follows Markovnikov regioselectivity and is anti-stereospecific, proceeding by a mechanism similar to other electrophilic addition reactions.
Copyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
You are on page 1of 6
4.
5 Oxymercuration [Hg(OAc)2/ROH]
Oxymercuration-demercuration is the reaction
when alkene treated with mercuric salt [Hg(OAc)2]
(Mercury II acetate) and alcohol [ROH], and ether is
formed.
Mercuration is an electrophilic addition of a mercuric salt to an
alkene, and the resulting compound is an alkyl-mercury
derivate, from which the mercury can be removed in a
subsequent step.
And the overall reaction is called oxymercuration-
demercuration.
Oxymercuration is anti stereospecific and regioselective.
This outcome implies a mechanism similar to that for
electrophilic addition reactions.
Mechanism of Oxymercuration-Demercuration
1. The mercury reagent initially dissociates to give
an acetate ion and a cation mercury species.
2. This mercury cation then attacks an alkene double bond,
furnishing a mercurinium ion.
3. Markovnikov rule regioselectivity: The alcohol that is present
attacks the more substituted carbon to give an alkylmercuric
acetate intermediate.
4. Demercuration: Replacement of mercury by hydrogen is
achieved by sodium borohydride reduction through a complex and
only incompletely understood mechanism. It is not stereospecific.
You might also like
- Omd - IIT Chemistry Tips, Advanced Organic Chemistry TipsDocument5 pagesOmd - IIT Chemistry Tips, Advanced Organic Chemistry TipsPawan BabelNo ratings yet
- Alkene ReactionsDocument34 pagesAlkene ReactionsIamKasiraporn MahanilNo ratings yet
- Important MechanismsDocument11 pagesImportant Mechanismsyimisa2927No ratings yet
- Electrophilic Addition of Alkenes NotesDocument17 pagesElectrophilic Addition of Alkenes NotesAnanda Vijayasarathy100% (1)
- LECTURE 6C The Chemistry of Alkenes and Alkynes CME 2023Document37 pagesLECTURE 6C The Chemistry of Alkenes and Alkynes CME 2023LinearNo ratings yet
- Reactions of Organic FamiliesDocument5 pagesReactions of Organic FamiliesJennifer Carolina Rosales NoriegaNo ratings yet
- Hydrohalogenation of Alkenes and Dehydrohalogenation of HaloalkanesDocument18 pagesHydrohalogenation of Alkenes and Dehydrohalogenation of HaloalkanesSyaza Ahmad JaisNo ratings yet
- Chapter 4 AlkeneDocument64 pagesChapter 4 AlkeneMELVINDO JACOBNo ratings yet
- Alkenes PDFDocument22 pagesAlkenes PDFIsuri VidyarathneNo ratings yet
- Alkanes have only strong σ bonds. Because the carbon and hydrogen atoms of an alkaneDocument6 pagesAlkanes have only strong σ bonds. Because the carbon and hydrogen atoms of an alkaneDebasish SharmaNo ratings yet
- On Oxymercuration and DemercurationDocument21 pagesOn Oxymercuration and DemercurationBapu ThoratNo ratings yet
- Organic ReactionsDocument44 pagesOrganic ReactionsJanhavi PatilNo ratings yet
- Lec 6C Alkyne Structure and ReactivityDocument37 pagesLec 6C Alkyne Structure and Reactivityseanroi.villas.lawNo ratings yet
- Adisi Elektrofilik Alkena AlkunaDocument37 pagesAdisi Elektrofilik Alkena Alkunatutik kharismayantiNo ratings yet
- Halogen Derivative of AlkaneDocument29 pagesHalogen Derivative of AlkaneDeepti Kaskar60% (5)
- AlkenesDocument16 pagesAlkenesAbhijeetNo ratings yet
- Organic Chemistry BKF1323: 2.2 AlkenesDocument55 pagesOrganic Chemistry BKF1323: 2.2 Alkenes0JTINGNo ratings yet
- 10 Ak Part 3Document4 pages10 Ak Part 3Shamanth MNo ratings yet
- Organic Chemistry Alkynes ReactionsDocument9 pagesOrganic Chemistry Alkynes ReactionsAnthony KwofieNo ratings yet
- Alkane Chemistry AssignmentDocument6 pagesAlkane Chemistry AssignmentNafees ImtiazNo ratings yet
- AlkaneDocument14 pagesAlkanebijansharma019No ratings yet
- Lecture 6 WordsDocument5 pagesLecture 6 WordsDonald LetsoNo ratings yet
- Hydrocarbons (Alkanes and Alkenes)Document16 pagesHydrocarbons (Alkanes and Alkenes)Soham NagNo ratings yet
- Week 11 AlkunaDocument54 pagesWeek 11 AlkunaNazuwa RosaNo ratings yet
- CH 7 7eDocument42 pagesCH 7 7eVy TranNo ratings yet
- Named ReactionDocument21 pagesNamed ReactionVijaya RajuNo ratings yet
- Elimination Reaction NotesDocument10 pagesElimination Reaction Notesseema yadavNo ratings yet
- Module 1 - Electrochemical EnergyDocument129 pagesModule 1 - Electrochemical EnergyknightruzelNo ratings yet
- Chapter 16 - Oxidation and ReductionDocument50 pagesChapter 16 - Oxidation and ReductionGeorgeNo ratings yet
- 4.2 Hydration of AlkenesDocument33 pages4.2 Hydration of AlkenesDawit BirhanuNo ratings yet
- Electrochemistry: (Tuesday, 8 May 2017)Document18 pagesElectrochemistry: (Tuesday, 8 May 2017)mipa amarNo ratings yet
- AdisiDocument107 pagesAdisiazizgagabNo ratings yet
- AlkenesDocument120 pagesAlkenesVidhan PatniNo ratings yet
- Advances in Organometallic Chemistry and Catalysis: The Silver / Gold Jubilee International Conference on Organometallic Chemistry Celebratory BookFrom EverandAdvances in Organometallic Chemistry and Catalysis: The Silver / Gold Jubilee International Conference on Organometallic Chemistry Celebratory BookArmando J. L. PombeiroRating: 5 out of 5 stars5/5 (1)
- Permeating & Mechanisms PDFDocument18 pagesPermeating & Mechanisms PDFShabnam Fatima SiddiquiNo ratings yet
- Alkane FunctionalizationFrom EverandAlkane FunctionalizationArmando J. L. PombeiroNo ratings yet
- Chapter 15 - HydrocarbonsDocument16 pagesChapter 15 - HydrocarbonsNabindra RuwaliNo ratings yet
- Mod 3Document21 pagesMod 3Taulant SheqerxhiuNo ratings yet
- 19.1.2 Electrolytic CellsDocument9 pages19.1.2 Electrolytic CellsakshayajagadeeshwarNo ratings yet
- Ch06 Lecture All PDFDocument89 pagesCh06 Lecture All PDFasyaanazNo ratings yet
- Alkyne Chemistry EEEDocument42 pagesAlkyne Chemistry EEEVictor MutugiNo ratings yet
- Niu Ejoc2020rewDocument15 pagesNiu Ejoc2020rewBálint NagyNo ratings yet
- Preparation and Reaction of Carboxylic AcidsDocument6 pagesPreparation and Reaction of Carboxylic AcidsIndhumathiNo ratings yet
- 6) Chapter 8 Alkenes and Alkynes IIDocument44 pages6) Chapter 8 Alkenes and Alkynes IIfarah_affandyNo ratings yet
- Report 2 ElectrolysisDocument19 pagesReport 2 ElectrolysisOmar SamirNo ratings yet
- Chapter - 13 Hydro CarbonDocument22 pagesChapter - 13 Hydro CarbonManan TyagiNo ratings yet
- Electrowinning and Electrorefining of Copper (Murdoch University)Document76 pagesElectrowinning and Electrorefining of Copper (Murdoch University)Victor100% (8)
- Photochemical Kinetics: Activated MoleculeDocument7 pagesPhotochemical Kinetics: Activated MoleculeCarl Jerome AustriaNo ratings yet
- Haloalkanes and Haloarenes NotesDocument10 pagesHaloalkanes and Haloarenes NotesArchanaa PadmavathiNo ratings yet
- IA 3 PD 1 Traffic LightDocument16 pagesIA 3 PD 1 Traffic LightEric Issel ThomasNo ratings yet
- GssDocument26 pagesGssvnikhar123100% (1)
- Transition Metals: Presented By: Supervised byDocument11 pagesTransition Metals: Presented By: Supervised bymaryam zaxoNo ratings yet
- Topic 4 - Electrochemistry - Student Version 20202021Document104 pagesTopic 4 - Electrochemistry - Student Version 20202021Farah CakeyNo ratings yet
- Mercury FulminateDocument11 pagesMercury FulminateAnoshän IndreswaränNo ratings yet
- Notes 02Document67 pagesNotes 02Christine FernandezNo ratings yet
- Freeradicals 171225065343Document58 pagesFreeradicals 171225065343Jawad HussainNo ratings yet
- HYDROCARBONS Plusone HssliveDocument13 pagesHYDROCARBONS Plusone HssliveAthulRKrishnanNo ratings yet
- Mercury Removal Using Al - Al Electrodes by ElectrocoagulationDocument7 pagesMercury Removal Using Al - Al Electrodes by ElectrocoagulationIJMERNo ratings yet
- Hydrocarbons PDFDocument12 pagesHydrocarbons PDFMayank ShahabadeeNo ratings yet
- Arene Chemistry: Reaction Mechanisms and Methods for Aromatic CompoundsFrom EverandArene Chemistry: Reaction Mechanisms and Methods for Aromatic CompoundsNo ratings yet
- Pharmacy Service Management For L-III COURSE SYLLABUSDocument2 pagesPharmacy Service Management For L-III COURSE SYLLABUSDawit BirhanuNo ratings yet
- Manage Pharm - Service Chapter-1Document34 pagesManage Pharm - Service Chapter-1Dawit BirhanuNo ratings yet
- Manage Pharm - Service Chapter 4 EDDocument27 pagesManage Pharm - Service Chapter 4 EDDawit BirhanuNo ratings yet
- Introduction To Pharmacy ManagementDocument27 pagesIntroduction To Pharmacy ManagementDawit BirhanuNo ratings yet
- Manage Pharm - Service Chapter-2Document43 pagesManage Pharm - Service Chapter-2Dawit BirhanuNo ratings yet
- Ethnobotanical KontaDocument15 pagesEthnobotanical KontaDawit BirhanuNo ratings yet
- University of Gondar College of Natural and Computational Sciences Department of BiologyDocument76 pagesUniversity of Gondar College of Natural and Computational Sciences Department of BiologyDawit Birhanu100% (1)
- Ketones Can Be Converted Into Esters Via The Insertion of An Oxygen Atom Baeyer-Villiger OxidationDocument3 pagesKetones Can Be Converted Into Esters Via The Insertion of An Oxygen Atom Baeyer-Villiger OxidationDawit BirhanuNo ratings yet
- 4.7 Hofmann RXNDocument4 pages4.7 Hofmann RXNDawit BirhanuNo ratings yet
- University of Gondar College of Natural and Computational Sciences Department of BiologyDocument76 pagesUniversity of Gondar College of Natural and Computational Sciences Department of BiologyDawit BirhanuNo ratings yet
- Structure Based Drug DesignDocument61 pagesStructure Based Drug DesignDawit BirhanuNo ratings yet
- Lay Armachiho EthnobotanyDocument88 pagesLay Armachiho EthnobotanyDawit BirhanuNo ratings yet
- 4.10 Epoxidation RXNDocument6 pages4.10 Epoxidation RXNDawit BirhanuNo ratings yet
- 4.2 Hydration of AlkenesDocument33 pages4.2 Hydration of AlkenesDawit BirhanuNo ratings yet
- 4.5 Oxidative cleveage-KMnO4 & O3Document21 pages4.5 Oxidative cleveage-KMnO4 & O3Dawit BirhanuNo ratings yet
- Proteins As Drug Targets: EnzymesDocument19 pagesProteins As Drug Targets: EnzymesDawit BirhanuNo ratings yet
- Presented By: Anand Sagar Tiwari M.Pharm (Second Semester)Document56 pagesPresented By: Anand Sagar Tiwari M.Pharm (Second Semester)Dawit BirhanuNo ratings yet
- 4.3 Alkyhalide PreparationDocument23 pages4.3 Alkyhalide PreparationDawit BirhanuNo ratings yet
- 4.1.1 Protic Vs Aprotic SolventDocument36 pages4.1.1 Protic Vs Aprotic SolventDawit BirhanuNo ratings yet
- Retrospective Evaluation of Single Patient InvestiDocument9 pagesRetrospective Evaluation of Single Patient InvestiDawit BirhanuNo ratings yet
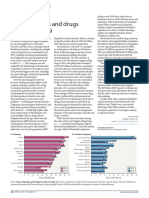
- Top Companies and Drugs by Sales in 2019: Market WatchDocument1 pageTop Companies and Drugs by Sales in 2019: Market WatchDawit BirhanuNo ratings yet
- 1.MedChem-L1 - 9Document327 pages1.MedChem-L1 - 9Dawit Birhanu100% (1)
- 1.0.clinical Study ProtocolDocument97 pages1.0.clinical Study ProtocolDawit BirhanuNo ratings yet
- 1.2.protocol V5Document73 pages1.2.protocol V5Dawit BirhanuNo ratings yet
- 1.1..clinical Study ProtocolDocument54 pages1.1..clinical Study ProtocolDawit BirhanuNo ratings yet