Professional Documents
Culture Documents
Biomolecules & Polymers (JEE Adv.) Exercise 2
Uploaded by
Amit0 ratings0% found this document useful (0 votes)
106 views19 pagesCopyright
© © All Rights Reserved
Available Formats
PPTX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
106 views19 pagesBiomolecules & Polymers (JEE Adv.) Exercise 2
Uploaded by
AmitCopyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
You are on page 1of 19
Chemistry
Biomolecules & Polymers
JEE Advanced
Exercise -2
Problem Solving
Course
www.etoosindia.com
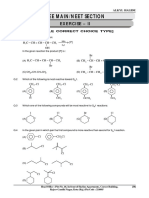
1. Which of the following pairs give positive Tollen’s
test ? [IIT 2004] (A) Glucose,
sucrose
(B) Glucose, fructose
(C) Hexanal, acetophenone
(D) Fructose, sucrose
2. The two forms of D–glucopyranose obtained from
the solution of D–glucose are called :
[IIT 2005]
(A) isomer (B) anomer
(C) epimer (D) enantomer
3. Column – I Column – II
[IIT 2007]
(A) Cellulose (P) natural polymer
(B) nylon – 6, 6 (Q) synthetic polymer
(C) protein (R) amide linkage
(D) sucrose (S) glycoside linkage
4. Cellulose upon acetylation with excess acetic
anhydride/H2SO4 (catalytic) gives cellulose
triacetate whose structure is [IIT 2008]
5. Among cellulose, poly (vinyl chloride), nylon and
natural rubber, the polymer in which the
intermolecular form of attraction is weakest is :
[IIT 2009] (A) nylon
(B) poly (vinyl chloride) (C) cellulose (D)
natural rubber
6. The correct statement(s) about the following
sugars X and Y is (are) [IIT 2009]
(A)X is a reducing sugar and Y is a non–reducing
sugar
(B)X is a non–reducing sugar and Y is a reducing
sugar
(C)The glucosidic linkages in X and Y are a and
b, respectively
(D)The glucosidic linkages in X and Y are b and
a, respectively
7. The correct statement about the following
disaccharide is [IIT 2010]
(A) Ring (a) is pyranose with a–glacosidic link
(B) Ring (a) is furanose with a–glycosidic link
(C) Ring (b) is furanoswe with a–glycosidic link
(D) Ring (b) is pyranose with b–glycosidic link
8. The following carbohydrate is [IIT 2011]
(A) a ketohexose (B) an aldohexose
(C) an a – furanose (D) an a–pyranose
9. The correct functional group X and the
reagent/reaction conditions Y in the following
scheme are [IIT 2011]
(A) X = COOCH3, Y = H2/Ni/heat
(B) X = CONH2, Y = H2/Ni/heat
(C) X = COHN2, Y = Br2/Naoh
(D) X = CN, Y = H2/Ni/heat
10. A tetrapeptide has – COOH group on alanine.
This produces glycine (Gly), valine (Val), phenyl
alanine (Phe) and alanine (Ala), on complete
hydrolysis. For this tetrapeptide, the number of
possible sequences (primary structures) with –
NH2 group attached to a chiral centre is
[JEE Advance 2013]
ONE OR MORE THAN ONE
11. The structure of D – (+) – glucose is
[JEE Advance 2015]
The structure of L–(–)–glucose is
SINGLE CHOICE QUESTIONS
12. On complete hydrogenation, natural rubber
produces [JEE Advance 2016]
(A) Ethylene–propylene copolyer
(B) Vulcanised rubber
(C) Polyproplene
(D) Polybutylene
You might also like
- Trends in properties of s-block elementsDocument84 pagesTrends in properties of s-block elementsPrakhar ShuklaNo ratings yet
- Solenoid Valve - TestDocument10 pagesSolenoid Valve - TestFausto100% (6)
- Alcohol & EtherDocument217 pagesAlcohol & EtherAmitNo ratings yet
- Making A Prallel Jaw Bar ClampDocument34 pagesMaking A Prallel Jaw Bar ClampHomayoon GeramifarNo ratings yet
- Astm A105 2021Document5 pagesAstm A105 2021Pedrito Calapucha100% (2)
- 3.AcidBases FinalDocument35 pages3.AcidBases FinalSoham RaneNo ratings yet
- Jupiter: Largest Planet in the Solar SystemDocument29 pagesJupiter: Largest Planet in the Solar SystemLakshmish GopalNo ratings yet
- Beckman Coulter HematologyDocument249 pagesBeckman Coulter HematologyIbrahim Ahmad100% (3)
- Stereoisomerism VKP SirDocument49 pagesStereoisomerism VKP SirSandeep ReddyNo ratings yet
- DPPs BOOKLET-2 - CHEMISTRY REVISIONDocument81 pagesDPPs BOOKLET-2 - CHEMISTRY REVISIONKushal RathoreNo ratings yet
- Biomolecules & Polymers Exercise 1Document90 pagesBiomolecules & Polymers Exercise 1Aditya ShahNo ratings yet
- 04 IsomerismDocument19 pages04 IsomerismSoham RaneNo ratings yet
- Halogen Derivatives PDFDocument32 pagesHalogen Derivatives PDFRaju Singh100% (1)
- Isomerism - Handwritten NotesDocument7 pagesIsomerism - Handwritten Notesgovind_galamNo ratings yet
- Part - I: Objective Questions: Section A: Geometrical IsomerismDocument10 pagesPart - I: Objective Questions: Section A: Geometrical IsomerismTejas pawarNo ratings yet
- 235practice Exam 2 AnswerDocument9 pages235practice Exam 2 Answernbobs7No ratings yet
- CLS Aipmt-19-20 XIII Che Study-Package-3 Level-1 Chapter-15 PDFDocument40 pagesCLS Aipmt-19-20 XIII Che Study-Package-3 Level-1 Chapter-15 PDFThavasimariselvam N100% (1)
- Resonance and Inductive Effects in Organic ChemistryDocument36 pagesResonance and Inductive Effects in Organic Chemistryeagl33yeNo ratings yet
- Gas LawDocument6 pagesGas LawrambabuNo ratings yet
- GOC Practice Assignment CHCDocument26 pagesGOC Practice Assignment CHCjanviNo ratings yet
- Salt Analysis (Theory) - EngDocument28 pagesSalt Analysis (Theory) - Engjoxis70026100% (1)
- Coordination Chemistry JEE AdvancedDocument44 pagesCoordination Chemistry JEE AdvancedKartikey SharmaNo ratings yet
- Synthesis, Characterization of New Schiff Base and Some Metal Complexes Derived From Glyoxylic Acid and O-PhenylenediamineDocument12 pagesSynthesis, Characterization of New Schiff Base and Some Metal Complexes Derived From Glyoxylic Acid and O-PhenylenediamineAndzhiita SaampeerNo ratings yet
- Practice 24 - PDB Cloning and Relocation Using DBCADocument11 pagesPractice 24 - PDB Cloning and Relocation Using DBCALogis M100% (1)
- Pricing Determination Procedure PDFDocument62 pagesPricing Determination Procedure PDFTaslimNo ratings yet
- Organic Chemistry Guided Revision Plan-Score AdvancedDocument4 pagesOrganic Chemistry Guided Revision Plan-Score AdvancedNamchrahsiNo ratings yet
- Alcohols WsDocument5 pagesAlcohols WsVedanta DesikNo ratings yet
- Alkyl and Aryl Halides - DPP-05 - Alkyl and Aryl halides-DPP-05 - (NEET) Lakshay BatchDocument4 pagesAlkyl and Aryl Halides - DPP-05 - Alkyl and Aryl halides-DPP-05 - (NEET) Lakshay BatchAryan SinghNo ratings yet
- Previous Years Board Question of Alkyl and Aryl Halide PDFDocument13 pagesPrevious Years Board Question of Alkyl and Aryl Halide PDFKomal TripathiNo ratings yet
- Goc and Isomerism Notes - PMD - 1 PDFDocument46 pagesGoc and Isomerism Notes - PMD - 1 PDFrutvik bhoraniyaNo ratings yet
- Class - Xii Subject - ChemistryDocument70 pagesClass - Xii Subject - ChemistryYash TandonNo ratings yet
- Alcohol Phenol Ether PDFDocument38 pagesAlcohol Phenol Ether PDFsjahsnjNo ratings yet
- Solved Example: Chemistry For Neet & AiimsDocument24 pagesSolved Example: Chemistry For Neet & AiimsAnup KNo ratings yet
- 12 Chemistry Impq CH10 Haloalkanes and Haloarenes 02Document47 pages12 Chemistry Impq CH10 Haloalkanes and Haloarenes 02Swaroop SurendraNo ratings yet
- Chemistry ProjectDocument17 pagesChemistry ProjectANTARO MASSENNo ratings yet
- Amines PDFDocument34 pagesAmines PDFRam KhannaNo ratings yet
- Nomenclature - DPP OkDocument12 pagesNomenclature - DPP Oknawazishmd819100% (1)
- DPP GoccccDocument10 pagesDPP GoccccMayur Khichi0% (1)
- Iit Jee Chemistry DPP by :pjoyDocument3 pagesIit Jee Chemistry DPP by :pjoyPrakash Joy50% (4)
- Pdf-Haloalkanes and HaloarenesDocument159 pagesPdf-Haloalkanes and HaloarenesOmkar Singh Shekhawat100% (2)
- Coordination Compound: IIT-JEE 2013Document50 pagesCoordination Compound: IIT-JEE 2013Utkarsh Agarwal100% (1)
- Name Reaction Reagent Assignment PDFDocument21 pagesName Reaction Reagent Assignment PDFSandipan SahaNo ratings yet
- Telegram Channel for JEE ToppersDocument267 pagesTelegram Channel for JEE ToppersGyanesh DwivediNo ratings yet
- Carbonyl Compounds Question Paper For JEE Advanced 2019Document6 pagesCarbonyl Compounds Question Paper For JEE Advanced 2019misostudyNo ratings yet
- Chem Academy: Chemical BondingDocument4 pagesChem Academy: Chemical BondingEmraan EmmiNo ratings yet
- CBSE Class 12 Alcohol Phenol and Ether Study NotesDocument378 pagesCBSE Class 12 Alcohol Phenol and Ether Study NotesV T PRIYANKANo ratings yet
- Topic: Wacker Process Presented To: DR - Abid Zia Presented By: Neha TariqDocument15 pagesTopic: Wacker Process Presented To: DR - Abid Zia Presented By: Neha Tariqneha tariqNo ratings yet
- Adichemistry Online Coaching Sample 1 PDFDocument13 pagesAdichemistry Online Coaching Sample 1 PDFMeenakshi GaurNo ratings yet
- CBSE Sample Question Papers For Class 12 Chemistry 2020Document16 pagesCBSE Sample Question Papers For Class 12 Chemistry 2020Emtiaz AnsariNo ratings yet
- Dipole Moments in Organic CHEMISTRYDocument18 pagesDipole Moments in Organic CHEMISTRYBalraj Dhillon100% (2)
- C N Et - Set - Gate - Tifr: Question Bank Organometallic ChemistryDocument17 pagesC N Et - Set - Gate - Tifr: Question Bank Organometallic ChemistryKartik RanaNo ratings yet
- IsomerismDocument62 pagesIsomerismsubesinghNo ratings yet
- Photosynthesis in Higher PlantsDocument25 pagesPhotosynthesis in Higher PlantsRaichal P BijuNo ratings yet
- Chemistry Class 11 AssignmentDocument5 pagesChemistry Class 11 AssignmentDON'T CRAMNo ratings yet
- Organic - Reagents FinalDocument27 pagesOrganic - Reagents FinalSankar AdhikariNo ratings yet
- Anic Chemistry Carbonyl CompoundsDocument6 pagesAnic Chemistry Carbonyl Compoundseamcetmaterials100% (1)
- Umpolung reactivity: methods for interchanging carbonyl donor and acceptor reactivityDocument28 pagesUmpolung reactivity: methods for interchanging carbonyl donor and acceptor reactivitymeauna100% (1)
- Haloalkanes and Haloarenes Class 12 Chemistry MCQs PDFDocument33 pagesHaloalkanes and Haloarenes Class 12 Chemistry MCQs PDFSanjana Sanjay100% (1)
- Model Questions On U-12, 13 & 14Document12 pagesModel Questions On U-12, 13 & 14kadedoxNo ratings yet
- 5.surface Chemistry Final 4-3-2014 PDFDocument16 pages5.surface Chemistry Final 4-3-2014 PDFArinjayNo ratings yet
- IIT-JEE CRASH COURSE TOPIC ISOMERISM REACTION MECHANISMDocument14 pagesIIT-JEE CRASH COURSE TOPIC ISOMERISM REACTION MECHANISMSachin DedhiaNo ratings yet
- DPP 01 Gaseous State JH Sir-3583Document11 pagesDPP 01 Gaseous State JH Sir-3583Shivam Kumar75% (4)
- Alkene DPPDocument20 pagesAlkene DPPKalyan ReddtNo ratings yet
- Acidity Basicity QuestionDocument16 pagesAcidity Basicity QuestionPriyÃnka KumariNo ratings yet
- Polytechnic TRB Syllabus of ChemistryDocument4 pagesPolytechnic TRB Syllabus of ChemistrysanjeevNo ratings yet
- CSIR UGC NET Model Question Papers Chemical SciencesDocument32 pagesCSIR UGC NET Model Question Papers Chemical SciencesShiksha PortalNo ratings yet
- Revision Notes For Class 12 CBSE Chemistry, Alcohols, Phenols and Ethers - TopperlearningDocument10 pagesRevision Notes For Class 12 CBSE Chemistry, Alcohols, Phenols and Ethers - TopperlearningRishabh Bhandari67% (3)
- Auto Secure - Liability Only PolicyDocument4 pagesAuto Secure - Liability Only PolicyAmitNo ratings yet
- Drawing Block TitleDocument18 pagesDrawing Block TitleDanister GladwinNo ratings yet
- Iupac 1Document37 pagesIupac 1shodhan shettyNo ratings yet
- Grignard Reagent & DiazotisationDocument121 pagesGrignard Reagent & DiazotisationAditya ShahNo ratings yet
- Carbonyl CompoundDocument197 pagesCarbonyl CompoundAmitNo ratings yet
- Biomolecules & Polymers (JEE Adv.) Exercise 3Document17 pagesBiomolecules & Polymers (JEE Adv.) Exercise 3AmitNo ratings yet
- Biomolecules & Polymers (JEE Adv.) Exercise 1Document19 pagesBiomolecules & Polymers (JEE Adv.) Exercise 1Aditya ShahNo ratings yet
- Alkyl Halide THEORY 1-28Document28 pagesAlkyl Halide THEORY 1-28Aditya ShahNo ratings yet
- Exercise - I: CH CH OHDocument5 pagesExercise - I: CH CH OHAditya ShahNo ratings yet
- NEET II 38 - 56 (Exercise 2)Document19 pagesNEET II 38 - 56 (Exercise 2)AmitNo ratings yet
- New Et CH 3 and CH4 PDFDocument63 pagesNew Et CH 3 and CH4 PDFAmitNo ratings yet
- Chemistry: Carbene & NitreneDocument188 pagesChemistry: Carbene & NitreneAmitNo ratings yet
- Isomerism Theory 1-27Document32 pagesIsomerism Theory 1-27Aditya ShahNo ratings yet
- Carbonyl CompoundDocument197 pagesCarbonyl CompoundAmitNo ratings yet
- Iupac 1Document37 pagesIupac 1shodhan shettyNo ratings yet
- System SimulationDocument304 pagesSystem SimulationAmitNo ratings yet
- Exam - Inhinyero - Student Portal - DesignDocument30 pagesExam - Inhinyero - Student Portal - DesignJacob SantosNo ratings yet
- Giant Salt Basin in Peru OverlookedDocument18 pagesGiant Salt Basin in Peru OverlookedFred LuqueNo ratings yet
- The Difference Between The Academic Performance of Woking and NonDocument8 pagesThe Difference Between The Academic Performance of Woking and NonMr.nutshell CoronelNo ratings yet
- AC800F F2K Connect B1 6.2 CONFIGURATIONDocument43 pagesAC800F F2K Connect B1 6.2 CONFIGURATIONassessorNo ratings yet
- Apex Series 5000 7000 Bill Acceptor Manual PDFDocument17 pagesApex Series 5000 7000 Bill Acceptor Manual PDFFidelRomasantaNo ratings yet
- Jee FS MT-D 28-12-2023 JM PaperDocument16 pagesJee FS MT-D 28-12-2023 JM PaperbusinesspratssyyNo ratings yet
- Assignment-5 Enmt610029 Welding ANDARADHI NARARYA/1206291992Document8 pagesAssignment-5 Enmt610029 Welding ANDARADHI NARARYA/1206291992Andaradhi NararyaNo ratings yet
- EED 5 Unit 8Document45 pagesEED 5 Unit 8Reinaliza FerraroNo ratings yet
- Controlled Atmosphere StorageDocument9 pagesControlled Atmosphere StorageAnaniah BlessingNo ratings yet
- Thailand International Mathematical Olympiad Syllabus: Kindergarten GroupDocument5 pagesThailand International Mathematical Olympiad Syllabus: Kindergarten GroupEly SoemarniNo ratings yet
- WOCD-0306-02 Rotary Drilling With Casing - A Field Proven Method of Reducing Wellbore Construction CostDocument7 pagesWOCD-0306-02 Rotary Drilling With Casing - A Field Proven Method of Reducing Wellbore Construction CostMile SikiricaNo ratings yet
- K-Means Clustering in PHP: Group Unknown Data into K Number of ClustersDocument4 pagesK-Means Clustering in PHP: Group Unknown Data into K Number of ClustersMuhammad Iqbal100% (1)
- Controlling Water Level Using SensorsDocument3 pagesControlling Water Level Using SensorsnandeeshNo ratings yet
- Syllabus PHYS110 Fall2015 PDFDocument4 pagesSyllabus PHYS110 Fall2015 PDFPadmaja SundaramNo ratings yet
- Chapter 4Document44 pagesChapter 4Ping LeungNo ratings yet
- Topographic Map of VenusDocument1 pageTopographic Map of VenusHistoricalMapsNo ratings yet
- Difequa Higherorderde PDFDocument119 pagesDifequa Higherorderde PDFJj DaneNo ratings yet
- Amacs LEBM0079-00Document4 pagesAmacs LEBM0079-00mustafasenbagci3693No ratings yet
- Computer Aided Design and FE Analysis of A PM BLDG Hub MotorDocument6 pagesComputer Aided Design and FE Analysis of A PM BLDG Hub MotorDebarati DamNo ratings yet
- Activity 1Document13 pagesActivity 1Marving ZNo ratings yet
- Mouna HezbriDocument106 pagesMouna HezbriMuhammedNo ratings yet
- Hysteretic Energy DissipationDocument22 pagesHysteretic Energy Dissipationdharma raj upadhyayaNo ratings yet
- ThinkServer TD350 - Product GuideDocument27 pagesThinkServer TD350 - Product GuideRaYa DiawNo ratings yet