Professional Documents
Culture Documents
Hematopoiesis and Erytherokinitics
Uploaded by
Shahid F.S.D.0 ratings0% found this document useful (0 votes)
9 views20 pagesErythrokinetics
Original Title
RBC Kinetics
Copyright
© © All Rights Reserved
Available Formats
PPTX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentErythrokinetics
Copyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
9 views20 pagesHematopoiesis and Erytherokinitics
Uploaded by
Shahid F.S.D.Erythrokinetics
Copyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
You are on page 1of 20
Hematopoiesis and Erytherokinitics
THE ERYTHRON
• widely dispersed mass of erythroid cells includes circulating
erythrocytes & bone marrow precursor, progenitor, stem cells.
• Function: oxygen transport mediated by hemoglobin.
• Hb consists of heme & globin (tetramer)
• Each heme have Fe2+.
• Globin chain of specific sequence amino acid attached to each heme
group.
• Hb. a tetramer, containing 4 heme units & 4 globin chains.
HEME SYNTHESIS
• Heme synthesis unidirectional & irreversible, controlled at first step by
enzyme δ-aminolevulinic acid synthase, synthesis is controlled by
negative feedback from heme conc. within erythrocyte.
• Lead inhibits delivery of iron to site of ferrochelatase activity.
• Chloramphenicol may inhibit heme synthesis.
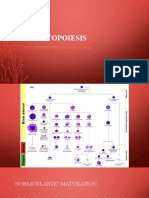
Hematopoiesis
Hematopoiesis
• Stem cells, progenitor cells, & precursor cells
• Stem cells
• Pluripotential & multipotential stem cells (CFU-GEMM or CD34+ cells)
• Capacity for self-renewal & differentiate into progenitor cells.
• Controlled by growth-promoting stimuli produced by marrow stromal
cells.
• Growth factors & cytokines involved (SCF, IL-3, IL-9, IL-11, & Epo)
• When a stem cell differentiates, loses some of its ability to self-
replicate & some of its potentiality.
Progenitor cells
• Multi potential progenitor cells have capability of differentiating into
more than one cell line
• (e.g., CFU-GEMM differentiate into granulocytes, erythrocytes,
monocytes, or megakaryocytes).
• Uni-potential progenitor CFU-E differentiate into erythroid cells
• Limited capacity for self renewal & differentiate into precursor cells of
various cell lines.
• not recognizable morphologically, resemble small lymphocytes.
Precursor cells
• Have no capacity for self-renewal but proliferate while differentiating
into mature, functional cells.
• First cells that can be recognized as members of a particular cell line.
• Erythropoiesis occurs extra-vascularly in bone marrow parenchyma.
Hematopoiesis
Morphologic changes during maturation from rubriblast to mature RBCs
• Cells become smaller.
• Nuclei become smaller & chromatin aggregated:
• Cell division stops in late rubricyte stage when conc. of Hb deposited
• Nucleus extruded at metarubricyte state, a reticulocyte formed in
mammals.
• Avian reticulocytes & mature erythrocytes retain their nuclei.
• Cytoplasmic color changes from blue to orange as Hb is formed &
RNA lost.
• Reticulocytes & RBCs migrate into venous sinus of bone marrow
• through transient apertures in endothelial cell cytoplasm.
• Reticulocytes remain in bone marrow for 2-3days before release &
ultimately mature in peripheral blood or spleen.
• Time from stimulation of progenitor cell to reticulocytes released
approx. five days.
• Starting with the rubriblast, three to five divisions produce eight to 32
differentiated cells.
• Bone marrow has capacity to increase erythropoiesis.
• RBCs production increased up to 7 times normal rate in humans,
• providing necessary stimulation & nutrients available
. Regulation of erythropoiesis
• 1. Erythropoietin (Epo)
• produced by peritubular interstitial cells of kidney in response to
hypoxia,
• 10% to 15% of Epo production by specific hepatocytes
• Actions of Epo
• Inhibition of apoptosis of newly formed progenitor cells &
prorubricytes, allowing them to differentiate into mature RBCs
• Stimulation of Hb. synthesis in already dividing erythroid cells.
2.Interleukin-3 (IL-3) & colony-stimulating factors (GM-CSF and G-CSF).
• IL-3: by activated T-lymphocytes;
• CSF: by activated T-lymphocytes, macrophages, endothelial cells, &
fibroblasts; monocytes, neutrophils,
• Action:
• Stimulate multiplication of a primitive erythroid
• Progenitor cell, BFU-E, & its differentiation into CFU-E progenitor cell.
• BFU-E progenitor cell insensitive to Epo stimulation alone.
• 3. Androgens
• Increase Epo release.
• Estrogens and corticosteroids decrease Epo release, not clinically
significant.
• Thyroid and pituitary hormones
• alter tissue demands for oxygen, thereby changing requirement for
erythropoiesis.
ERYTHROCYTE DESTRUCTION
• Aver. RBCs lifespan 120 days
• Aging of RBCs due to changes in enzyme content & cell membrane
structure that make cells less capable of survival & subject to removal
by spleen.
• In health, senescent RBCs removed from circulation by two routes.
• Phagocytosis: macrophages major route of senescent erythrocyte
removal
• Within phagosome, RBC releases its Hb, split into heme & globin.
• Globin: break into amino acids & reutilized.
• Iron released from heme by heme oxygenase, forming CO & biliverdin
• Biliverdin reduced by biliverdin reductase to bilirubin, excreted into
blood,
• Bilirubin binds with albumin for transport to liver.
Intravascular phagocytosis
• Release of Hb into plasma, minor route of senescent RBCs removal
• Free Hb in plasma binds to α2-globulin, haptoglobin.
• Hemoglobinhaptoglobin complex cleared from plasma by liver,
preventing loss of Hb in urine.
• Enough haptoglobin bind 150 mg/dL of Hb
• Plasma appears pink to red when 50 to 100 mg/dL of Hb present;
discoloration of plasma precedes hemoglobinuria.
• In health, plasma discoloration not observed.
• If intravascular lysis is excessive, serum haptoglobin may become
saturated.
• Free Hb then dissociates into dimers, which can pass glomerular
filter, which does not occur in health.
• With time, free Hb in plasma is oxidized to methemoglobin, which
dissociates to free ferriheme, which complexes with β-globulin,
hemopexin
You might also like
- DrMercola SiimLandandJamesDiNicolantonio TheImmunityFixDocument28 pagesDrMercola SiimLandandJamesDiNicolantonio TheImmunityFixRocco LamponeNo ratings yet
- Characteristics and Functions of Blood ComponentsDocument51 pagesCharacteristics and Functions of Blood ComponentssamayaNo ratings yet
- IMMUNITY: A Concise Guide to Innate and Acquired ImmunityDocument190 pagesIMMUNITY: A Concise Guide to Innate and Acquired ImmunityFathima100% (1)
- 1 BloodDocument77 pages1 BloodVisaNathan100% (1)
- Cultivation of MicroorganismsDocument27 pagesCultivation of MicroorganismsLyndz Lee93% (14)
- Blood Cells, Immunity and Blood ClottingDocument65 pagesBlood Cells, Immunity and Blood Clottingmunaamuummee100% (1)
- ErythropoisisDocument47 pagesErythropoisisDisha SuvarnaNo ratings yet
- Red Blood Cells, Anemia, and PolycythemiaDocument7 pagesRed Blood Cells, Anemia, and PolycythemiaShi no Me100% (1)
- Bio 2 Quiz ReviewerDocument2 pagesBio 2 Quiz ReviewerAlexandra RoderoNo ratings yet
- HemopoesisDocument31 pagesHemopoesisChandra Shinoda100% (2)
- A Colour Atlas of Haematological Cytology by Hayhoe, PGDocument373 pagesA Colour Atlas of Haematological Cytology by Hayhoe, PGC0ghi86% (7)
- Red Blood Cells, Functions, Diseases A Simple Guide To The Condition, Diagnosis, Treatment, And Related ConditionsFrom EverandRed Blood Cells, Functions, Diseases A Simple Guide To The Condition, Diagnosis, Treatment, And Related ConditionsNo ratings yet
- BloodDocument5 pagesBloodChelsie NicoleNo ratings yet
- Erythrocyte Function, Metabolism, Production, BreakdownDocument20 pagesErythrocyte Function, Metabolism, Production, BreakdownAbdullah AzeemNo ratings yet
- Anatomy and Physiology of BLOOD: Preet KaurDocument37 pagesAnatomy and Physiology of BLOOD: Preet KaurPreet KaurNo ratings yet
- HemopoiesisDocument53 pagesHemopoiesisnav_malhiNo ratings yet
- LEC 5 RBC METABOLISM AND Membrane StructureDocument30 pagesLEC 5 RBC METABOLISM AND Membrane StructureFrancis ValdezNo ratings yet
- EritrositDocument12 pagesEritrositNatasya HerinNo ratings yet
- Plasma and Blood FunctionsDocument63 pagesPlasma and Blood Functionsحكيم العجلانNo ratings yet
- BloodDocument76 pagesBloodKerby Dela Fuente Alison100% (1)
- Hematology 2020Document63 pagesHematology 2020odiodi57No ratings yet
- Functions and Properties of Blood - Plasma - Blood Cell Production - Erythrocytes - Blood Types - Leukocytes - Hemostasis (Stoppage of Bleeding)Document47 pagesFunctions and Properties of Blood - Plasma - Blood Cell Production - Erythrocytes - Blood Types - Leukocytes - Hemostasis (Stoppage of Bleeding)vanderphysNo ratings yet
- Haemopoetic SystemDocument73 pagesHaemopoetic SystemManjunathNo ratings yet
- Physiology of Blood: Ratna Kusumawati Department of Physiology, Faculty of Medicine Universitas Sebelas Maret SurakartaDocument65 pagesPhysiology of Blood: Ratna Kusumawati Department of Physiology, Faculty of Medicine Universitas Sebelas Maret SurakartaRizal AbdurrahmanNo ratings yet
- Physiology of Blood: Ratna Kusumawati Department of Physiology, Faculty of Medicine Universitas Sebelas Maret SurakartaDocument60 pagesPhysiology of Blood: Ratna Kusumawati Department of Physiology, Faculty of Medicine Universitas Sebelas Maret SurakartaZidaneNo ratings yet
- RBC SeminarDocument80 pagesRBC SeminarSourav Ron BoseNo ratings yet
- Topics of This Lecture: RBC: - Structural Characteristics - Hemoglobin - Erythropoiesis - Erythrocytes DestructionDocument23 pagesTopics of This Lecture: RBC: - Structural Characteristics - Hemoglobin - Erythropoiesis - Erythrocytes DestructionHashim GhazoNo ratings yet
- Physiology (Unit 3)Document308 pagesPhysiology (Unit 3)Ahmed HashmiNo ratings yet
- HematopoiesisDocument71 pagesHematopoiesisTalent AshjayNo ratings yet
- Erythropoiesis Lec 1-PhysioDocument31 pagesErythropoiesis Lec 1-PhysioChandrani MadhiraNo ratings yet
- Red Blood Cells Lecture 4Document33 pagesRed Blood Cells Lecture 4Panashe MawaroNo ratings yet
- Blood PhysiologyDocument17 pagesBlood Physiologymuhammad sadiqNo ratings yet
- Blood Functions and CompositionDocument75 pagesBlood Functions and CompositionTina MinottNo ratings yet
- PSG 712 Blood-1Document54 pagesPSG 712 Blood-1preciousNo ratings yet
- The Essence of Life: A Concise Overview of Blood Composition and FunctionsDocument46 pagesThe Essence of Life: A Concise Overview of Blood Composition and FunctionsGrape JuiceNo ratings yet
- Erythropoiesis and AnaemiaDocument42 pagesErythropoiesis and AnaemiaKaylee NesbitNo ratings yet
- LEC3Document26 pagesLEC3Jessica StewartNo ratings yet
- Lecture7 - ErythropoiesisDocument31 pagesLecture7 - ErythropoiesisMarshina KunhammedNo ratings yet
- Blood: Erythropoeisis by U.SivakumarDocument41 pagesBlood: Erythropoeisis by U.SivakumarAkash JaatNo ratings yet
- Understanding the Composition and Functions of BloodDocument30 pagesUnderstanding the Composition and Functions of BloodPriyanka RameshNo ratings yet
- ERYTHROPOISISDocument22 pagesERYTHROPOISISW.F KareemNo ratings yet
- HematologyDocument38 pagesHematologyMichelle San Miguel FeguroNo ratings yet
- BloodDocument49 pagesBloodDale TelgenhoffNo ratings yet
- Red Cell.1Document25 pagesRed Cell.1Jude ChinecheremNo ratings yet
- Formed Elements: Drsallama Hamid Consultant Lecturer DoctorDocument7 pagesFormed Elements: Drsallama Hamid Consultant Lecturer DoctorAhmed Ali AssafNo ratings yet
- Physiology of BloodDocument73 pagesPhysiology of BloodAggamemnon populosNo ratings yet
- CVS BloodDocument39 pagesCVS BloodMohan GuptaNo ratings yet
- Erythropoiesis DPT SEM.1Document29 pagesErythropoiesis DPT SEM.1Hifza KhanNo ratings yet
- Blood Performs A Number of Functions Dealing WithDocument52 pagesBlood Performs A Number of Functions Dealing Withعلي العراقي فاديNo ratings yet
- Physiology Lecture 2: RBC S Characteristics, Function and FormationDocument20 pagesPhysiology Lecture 2: RBC S Characteristics, Function and FormationdarkboiNo ratings yet
- Blood: Group 1Document25 pagesBlood: Group 1Arian May MarcosNo ratings yet
- ErytropoiesisDocument23 pagesErytropoiesiswarda farooqNo ratings yet
- Next 4 Lectures:: Cardiovascular SystemDocument46 pagesNext 4 Lectures:: Cardiovascular SystemRevanth VejjuNo ratings yet
- Hematopoiesis: Part 2: Erythropoiesis and LeukopoiesisDocument62 pagesHematopoiesis: Part 2: Erythropoiesis and LeukopoiesisJohn Marie IdavaNo ratings yet
- RBC Characteristics, Anemia, PolycythemiaDocument20 pagesRBC Characteristics, Anemia, PolycythemiaAreej TariqNo ratings yet
- BloodDocument20 pagesBloodAdam PrabowoNo ratings yet
- BLOOD Update-1Document56 pagesBLOOD Update-1ahmadfadi343No ratings yet
- Red Blood Cells (Erythrocytes) LectureDocument41 pagesRed Blood Cells (Erythrocytes) LecturePhilip Abayomi VincentNo ratings yet
- IlovemymomDocument149 pagesIlovemymomREYSYMAE CABRALNo ratings yet
- 2020 Red Blood Cells (Erythrocytes)Document33 pages2020 Red Blood Cells (Erythrocytes)Raymon Villagonzalo TocoyoNo ratings yet
- Erythropoiesis and ErythropoietinDocument29 pagesErythropoiesis and ErythropoietinmaduksjaycryptoNo ratings yet
- RBC DISORDERS Part I VR 2022Document63 pagesRBC DISORDERS Part I VR 2022victoria tavasNo ratings yet
- Hematologic SystemDocument33 pagesHematologic SystemSteffanie TalaueNo ratings yet
- RBCS Synthesis (Autosaved)Document30 pagesRBCS Synthesis (Autosaved)AFTAB SHAIKHNo ratings yet
- Summative Test: Science 9Document33 pagesSummative Test: Science 9JoyR.AlotaNo ratings yet
- The Raas: Renin ReleaseDocument4 pagesThe Raas: Renin ReleaseAziil LiizaNo ratings yet
- Immunity To Intracellular BacteriaDocument27 pagesImmunity To Intracellular BacteriaNoor NawawraNo ratings yet
- Report NaveenDocument3 pagesReport NaveennaveenNo ratings yet
- A Quantitative Multivariate Model of Human Dendritic Cell-T Helper Cell CommunicationDocument38 pagesA Quantitative Multivariate Model of Human Dendritic Cell-T Helper Cell CommunicationSurjeet SinghNo ratings yet
- Molbio TransesDocument24 pagesMolbio TransesJonahlyn RegidorNo ratings yet
- Biofilms in EntDocument4 pagesBiofilms in EntDrsiya MedfriendNo ratings yet
- Protists FungiDocument77 pagesProtists FungiEgga AndiniNo ratings yet
- The Preanalytical PhaseDocument24 pagesThe Preanalytical PhaseArturo Eduardo Huarcaya OntiverosNo ratings yet
- Evaluation and Management of Young Febrile Infants 2023Document12 pagesEvaluation and Management of Young Febrile Infants 2023Felipe MayorcaNo ratings yet
- Corynebacterium DiphtheriaDocument39 pagesCorynebacterium DiphtheriaSubhada GosaviNo ratings yet
- Study of Effect of Antibiotics On MicroorganismsDocument19 pagesStudy of Effect of Antibiotics On MicroorganismsAnshika SinghNo ratings yet
- Cuando El Silencio InterrumpeDocument4 pagesCuando El Silencio Interrumpemilnest reyesNo ratings yet
- Week 1 Experimental Biology Daily Lesson Plans Samples 1Document30 pagesWeek 1 Experimental Biology Daily Lesson Plans Samples 1Tijana TosicNo ratings yet
- Lesotho Standard Guidelines Essential MedicinesDocument254 pagesLesotho Standard Guidelines Essential MedicinesportosinNo ratings yet
- Single Cell ProteinDocument15 pagesSingle Cell Proteinsharma28No ratings yet
- Immunity Questions and AnswersDocument12 pagesImmunity Questions and Answerskumara guruparanNo ratings yet
- Kualitas Perairan Tambak Udang Berdasar Parameter MikrobiologiDocument14 pagesKualitas Perairan Tambak Udang Berdasar Parameter MikrobiologirurikaimudinNo ratings yet
- A and Others vs. National Blood AuthorityDocument45 pagesA and Others vs. National Blood AuthorityGeorge ConkNo ratings yet
- Cancer Research American Association For Cancer ResearchDocument1 pageCancer Research American Association For Cancer ResearchYousab ShirefNo ratings yet
- HHS Public Access: B Cell Targeted Therapies in Autoimmune DiseaseDocument15 pagesHHS Public Access: B Cell Targeted Therapies in Autoimmune DiseaseLestiNo ratings yet
- WTTH - Multidrug Resistant Urinary Tract Infection in A Dog 33066 ArticleDocument5 pagesWTTH - Multidrug Resistant Urinary Tract Infection in A Dog 33066 ArticleCabinet VeterinarNo ratings yet
- Review of Spiking Illness Syndrome of Broiler Chickens in Nepal 2008Document39 pagesReview of Spiking Illness Syndrome of Broiler Chickens in Nepal 2008Dr.Kedar Karki ,M.V.Sc.Preventive Vet.Medicine CLSU Philippines100% (1)
- Identification of Five Cytotoxicity-Related GenesDocument12 pagesIdentification of Five Cytotoxicity-Related GenesFiona TranNo ratings yet
- Chemical Carcinogenesis I and Ii: Michael Lea 2016Document31 pagesChemical Carcinogenesis I and Ii: Michael Lea 2016aasiyaNo ratings yet