Professional Documents
Culture Documents
Krypton Note
Uploaded by
Aung Phone Khant0 ratings0% found this document useful (0 votes)
10 views10 pagesCopyright
© © All Rights Reserved
Available Formats
PPTX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
10 views10 pagesKrypton Note
Uploaded by
Aung Phone KhantCopyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
You are on page 1of 10
History of krypton
• Krypton was discovered in Britain in 1898 by William Ramsay(1852-1916), a Scottish
chemist(discovered argon, helium, neon, krypton and xenon) and Morris William
Travers(1872-1861) a British chemist(discovered neon, krypton and xenon) discovered
krypton as the residue of evaporating almost all of the other components of liquid air.
• Krypton was discovered partially by accident, that is why the original name of the krypton is
“kryptos” meaning hidden that is derived from the Greek.
• The element is named after the Sun God, 'Kryptos'.
• Sir William Ramsay was a giant when it came to the discovery of various gases in his time.
Unlike what most people see or think about the air, it is not simple. We don’t just breath in
elements that keep us alive and vital for function in everyday life. Again, William Ramsay
was the one who pointed it out.
• William Ramsay was awarded the 1904 Nobel Prize in Chemistry for discovery of a series
of noble gases, including krypton.
• Krypton was once used as a source for obtaining artificial rubies, which were produced
by heating corundum with krypton and helium and then exposing it to ultraviolet light so
as to produce red glowing crystals of corundum, known as Ruby Fussoir, which became
very popular during 1930s and 1940s.
• In 1933, the world's first neon lamp using Krypton gas was made by Frederick Mosby &
James Heyrovsk at NELCO Labs in Illinois USA. This sign is still in use and can be seen at
the Northwestern University.
• From 1960 to 1983 the isotope krypton-86 was used to define the standard measure of
length. One metre was defined as exactly 1,650,763.73 wavelengths of a line in the
atomic spectrum of the isotope.
• In 2010, scientists discovered that under certain ultrashort pulsed laser conditions
krypton can emit powerful electrical discharges at ten times the peak power of any light
source.
Krypton structure
• Krypton is characterized by several sharp emission lines (spectral
signatures) the strongest being green and yellow. Krypton is one of
the products of uranium fission. Solid krypton is white and has a face-
centered cubic crystal structure, which is a common property of all
noble gases (except helium, which has a hexagonal close-packed
crystal structure).
Background information about Krypton
• Krypton is one among the noble gases found on the periodic table along with the likes of
helium, neon and argon.
• 4th period and 18 groups
• Chemical symbol = Kr
• Room temperature = gas
• Boiling point = -153.4 C
• Melting point = -157.4 C
• Oxidation no = 0,2
• Groups = 18 (noble gas or single gas)
• Electron per shells = 2,8,18,8
• Density = 3.733 g/liters
Krypton isotopes
• Natural krypton is a mixture of six stable isotopes: krypton-84 (56.99 percent),
krypton-86 (17.28 percent), krypton-82 (11.59 percent), krypton-83 (11.5 percent),
krypton-80 (2.29 percent), and krypton-78 (0.36 percent).
• Krypton 1- proton=36, neutron=48
• Krypton 2- proton=36, neutron=44
• Krypton 3- proton=36, neutron=46
• Krypton 4- proton=36, neutron=47
• Krypton 5- proton=36, neutron=48
• Krypton 6- proton=36, neutron=56
• Krypton doesn’t have allortropes.
Characteristics
• Krypton is noble gas and non-metal.
• Being a noble gas means that krypton does not react chemically with other elements or
compounds except fluorine gas.
• Krypton is chemically highly unreactive.
• Krypton is colorless, tasteless and odorless.
• Krypton was left after liquid air had nearly boiled away.
• Krypton was left in the residue after boiling away water, oxygen, nitrogen, helium and argon from
the sample of air.
• Krypton needs to be handled with care, since it can explode on contact with methane, hydrogen
or nitrogen.
• Krypton is one of the rarest gases in the Earth’s atmosphere. It makes up just 1 part per million by
volume. It is extracted by distillation of air that has been cooled until it is a liquid. But in stars in
abundant quantity like the sun.
Uses of Krypton
• The element can make an excellent substitute for neon in certain conditions since it emits a weaker glow but still has low temperature and long life
to its credit.
• When electrons are shot at krypton, it just bounces the electrons back and we see that as light. That is why when electrons interact with krypton atoms inside the
glass, they emit visible light.
• Besides, when krypton is under high pressure and low temperature and is exposed to an electric current, krypton emits a reddish-orange light and
shines with a smokey-white light, much like a fluorescent light bulb does. So, if you see any LED display boards with blue or white lettering on
them, they are probably using krypton gas for emitting light instead of regular LEDs that emit light when electricity passes through semiconductor
material. Therefore, that appears to be its glow in the dark and it is used in making sings which are fluoresecent and for lighting up stars in
theaters, singboards, airports etc.
• Though finding its use in creating beautiful lighting effects in movies, Krypton was also once used as an anesthetic. The famous German chemist
Victor Grignard discovered this property of krypton when he accidentally inhaled some of the gas while working on the synthesis of organic
magnesium compounds.
• Krypton is used commercially as a filling gas for energy-saving fluorescent lights. It is also used in some flash lamps used for high-speed
photography. the ultra-sensitive cameras used in space missions to study distant stars and galaxies are filled with krypton gas for cooling the image
sensors to cryogenic temperatures.
• Unlike the lighter gases in its group, it is reactive enough to form some chemical compounds. For example, krypton will react with fluorine to form
krypton fluoride. Krypton fluoride is used in some lasers.
• Radioactive krypton was used during the Cold War to estimate Soviet nuclear production. The gas is a product of all nuclear reactors, so the
Russian share was found by subtracting the amount that came from Western reactors from the total in the air.
• In medical, it is used to detect abnormal heart openings.
Health effects
• Krypton is a colorless, odorless gas. It is shipped as a liquid under its
own vapor pressure. Contact with the liquid may cause frostbite to
unprotected skin.
• krypton is non-toxic, but breathing too much of it can cause loss of
consciousness because of asphyxia since the oxygen levels drop
quickly when krypton is released into regular air. Inhalation in
excessive concentrations can result in dizziness, nausea, vomiting, loss
of consciousness, and death.
Interesting facts about Krypton
• Krypton 85 can be used to detect secret nuclear weapons research and production facilities.
• Krypton is used in the airport to flash airport runways or used as a approach lights. (produce
brighter light and fast response to the electric current)
• Krypton is one of the rarest gases.
• Krypton is used to detect abnormal heat openings.
• Krypton-83 is used in MRI of the respiratory system.
• Krypton is believed to be a non-toxic asphyxia-inducing agent. (asphyxia = lack of oxygen
suffocation) krypton displaces breathable air
• Krypton-86 was used to define a meter.
• Krypton-fluorine lasers produce pluses with 500 times the power of the entire U.S electrical grid.
• Krypton is used to fill in multi-pane windows to conserve the energy. (kr reduces the heat transfer)
• Concentration of kr-85 is 30% higher at the north pole than they are at south pole.
Funny facts about Krypton
• Helium, argon, neon, krypton, and helium walk into a LGBT bar. The bar keeper says: ‘’
Get out of here, we don’t want far right elements in here’’.
• What does kryptonite mean in love?
• (noun) someone you love but he or she always ignores your affection and thus breaks your heart.
• What is Superman’s krypton name?
• Kal-El. Superman was born on the doomed planet Krypton to scientists Jor-El and Lara. He was
given the Kryptonian name Kal-El at birth.
• Krypton is superman’s home planet.
• Superman is the most famous fictional character that uses kryptonite against evil forces.
It has been believed that kryptonite could separate Superman from his powers; however,
it's only an assumption since no such material has ever been actually found on earth.
You might also like
- How Is Mercury Used Today? Chemistry Book for Kids 9-12 | Children's Chemistry BooksFrom EverandHow Is Mercury Used Today? Chemistry Book for Kids 9-12 | Children's Chemistry BooksNo ratings yet
- Krypton: The Hidden Chemical ElementDocument3 pagesKrypton: The Hidden Chemical ElementirfanmNo ratings yet
- A Closer Look at Silicon - Chemistry Book for Elementary | Children's Chemistry BooksFrom EverandA Closer Look at Silicon - Chemistry Book for Elementary | Children's Chemistry BooksNo ratings yet
- Krypton and SupermanDocument2 pagesKrypton and SupermanNttc JrwNo ratings yet
- The Periodic Table PresentationDocument10 pagesThe Periodic Table PresentationSingh GurleenNo ratings yet
- Prsentation On Krypton-Ali Raza TariqDocument23 pagesPrsentation On Krypton-Ali Raza TariqM Ali SheikhNo ratings yet
- How yttrium is extracted and used in superconductors and lasersDocument11 pagesHow yttrium is extracted and used in superconductors and lasersBagas AdiansyahNo ratings yet
- 450 Actinides PDFDocument43 pages450 Actinides PDFAnonymous 1YB2jwCK3No ratings yet
- A Project in Progress-1Document2 pagesA Project in Progress-1Darlene GumahadNo ratings yet
- PlutoniumDocument8 pagesPlutoniumKevinNo ratings yet
- KRYPTONDocument2 pagesKRYPTONKeylord Thomas CasasNo ratings yet
- ZIRCONIUM1Document19 pagesZIRCONIUM1Anonymous C6Ym0ENo ratings yet
- BakerDocument9 pagesBakervikash722No ratings yet
- Transition Metals II: (General Chemistry)Document11 pagesTransition Metals II: (General Chemistry)Erica Bianca MirandaNo ratings yet
- Screenshot 2023-06-03 at 9.20.48 PMDocument33 pagesScreenshot 2023-06-03 at 9.20.48 PMDavid AkinnubiNo ratings yet
- Noble Gases GroupDocument11 pagesNoble Gases GroupRoselyn BunquinNo ratings yet
- Radioactivity & Nuclear Physics ExplainedDocument36 pagesRadioactivity & Nuclear Physics Explainedmuhammad umairNo ratings yet
- Group 8A Noble GasesDocument2 pagesGroup 8A Noble GasesJohn Carlo ElchicoNo ratings yet
- Chemistry of ChromiumDocument27 pagesChemistry of ChromiumFebrian IsharyadiNo ratings yet
- Adobe Scan 13 Dec 2023Document11 pagesAdobe Scan 13 Dec 2023souravpaultechNo ratings yet
- Everything You Need to Know About ChlorineDocument19 pagesEverything You Need to Know About ChlorinetandonrNo ratings yet
- Research Paper of Chemistry I: The Element SeleniumDocument17 pagesResearch Paper of Chemistry I: The Element SeleniumStevenNo ratings yet
- Elemental HangmanDocument20 pagesElemental HangmanAdithya VardhanNo ratings yet
- Titanium.: Chemical PropertiesDocument3 pagesTitanium.: Chemical PropertiesIna TamayoNo ratings yet
- Lecture 36 - Group 18 (8A) : 2P32 - Inorganic ChemistryDocument8 pagesLecture 36 - Group 18 (8A) : 2P32 - Inorganic ChemistryS K MishraNo ratings yet
- Chem CH2 F4 Swa PDFDocument10 pagesChem CH2 F4 Swa PDFfarhanNo ratings yet
- Element PresentationDocument7 pagesElement Presentationsierra meredithNo ratings yet
- Chlorine: This Powerpoint Was Brought To You by The The Atomic Symbol CLDocument17 pagesChlorine: This Powerpoint Was Brought To You by The The Atomic Symbol CLruchita-tandon-9182No ratings yet
- (133840589) Carbon & Its Compounds NewDocument28 pages(133840589) Carbon & Its Compounds NewAbhishek GuptaNo ratings yet
- Prot ActiniumDocument13 pagesProt ActiniummikkasNo ratings yet
- Uranium - Poster FactsDocument3 pagesUranium - Poster FactsLarissa IoannoniNo ratings yet
- StrontiumDocument4 pagesStrontiumKevinNo ratings yet
- Isotopes and IsotopyDocument16 pagesIsotopes and IsotopyJenny ChocNo ratings yet
- ChemistryDocument14 pagesChemistryNaveedNo ratings yet
- 10 Compounds of Carbon ChemistryDocument26 pages10 Compounds of Carbon ChemistryparveezNo ratings yet
- Chemistry of Chromium: Properties, Uses and CompoundsDocument47 pagesChemistry of Chromium: Properties, Uses and CompoundsFebrian IsharyadiNo ratings yet
- Nuclear Chemistry Discovery and ApplicationsDocument47 pagesNuclear Chemistry Discovery and ApplicationsEmman Revilla100% (3)
- Group 6A Elements1Document62 pagesGroup 6A Elements1Jake Carillo Basas EsmerNo ratings yet
- Atomic Theory and Radioactive DecayDocument16 pagesAtomic Theory and Radioactive DecayNooruddin SheikNo ratings yet
- 10 - Group 18 - Nobel GasesDocument10 pages10 - Group 18 - Nobel Gasesfriasereca22No ratings yet
- UntitledDocument36 pagesUntitledahsan shahNo ratings yet
- Noble GasesDocument25 pagesNoble GasesandreasaryasatyaNo ratings yet
- Chemical SymbolDocument4 pagesChemical SymbolFritzie Andrea TirolNo ratings yet
- Lecture 33 - Group 18 (8A) : 2P32 - Principles of Inorganic Chemistry Dr. M. PilkingtonDocument16 pagesLecture 33 - Group 18 (8A) : 2P32 - Principles of Inorganic Chemistry Dr. M. PilkingtonS K MishraNo ratings yet
- Chapter 3 Atoms and ElementsDocument31 pagesChapter 3 Atoms and Elementschitminthu560345No ratings yet
- The Workings of An Ancient Nuclear Reactor - Scientific AmericanDocument9 pagesThe Workings of An Ancient Nuclear Reactor - Scientific AmericanAmelia WebsterNo ratings yet
- Term One Week 1 - 3Document3 pagesTerm One Week 1 - 3raphael_chang2637No ratings yet
- David Radius Hudson Colorado 1995 LectureDocument9 pagesDavid Radius Hudson Colorado 1995 LectureDOMINO66No ratings yet
- Lesson 3 - Synthesis of Elements in The LaboratoryDocument62 pagesLesson 3 - Synthesis of Elements in The Laboratorytheresa balatico100% (1)
- JFA Jewelries Product ElementsDocument12 pagesJFA Jewelries Product ElementsJulliana AngNo ratings yet
- UraniumDocument6 pagesUraniumKevinNo ratings yet
- Fossils and Measurement of Half-LifeDocument7 pagesFossils and Measurement of Half-Lifeيونس حسينNo ratings yet
- UraniumDocument10 pagesUraniumKevinNo ratings yet
- The AtomDocument185 pagesThe AtomEmmerson Ramir EspanaNo ratings yet
- UraniumDocument19 pagesUraniumEdgar Apaza HuallpaNo ratings yet
- Allotropes: C Is One of The Best KnownDocument9 pagesAllotropes: C Is One of The Best Knowndwivedi_gaurav94No ratings yet
- ScienceDocument25 pagesSciencekajal kumariNo ratings yet
- Elementary Scientist 1Document4 pagesElementary Scientist 1sarayooNo ratings yet
- Emergence of Ideas About AtomDocument27 pagesEmergence of Ideas About AtomJosh Schultz0% (1)
- LetterDocument1 pageLetterAung Phone KhantNo ratings yet
- DailyRoutine (Table)Document1 pageDailyRoutine (Table)Aung Phone KhantNo ratings yet
- Biometric Scanners Advantagesand DisadvantagesDocument1 pageBiometric Scanners Advantagesand DisadvantagesAung Phone KhantNo ratings yet
- Document ElementsDocument6 pagesDocument ElementsAung Phone KhantNo ratings yet
- Stage 8 Physics (Answers)Document1 pageStage 8 Physics (Answers)Aung Phone KhantNo ratings yet
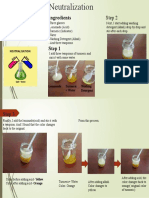
- Neutralization: How Acids and Alkalis ReactDocument2 pagesNeutralization: How Acids and Alkalis ReactAung Phone KhantNo ratings yet
- PlutoDocument2 pagesPlutoAung Phone KhantNo ratings yet
- How Rocks Weather Over TimeDocument2 pagesHow Rocks Weather Over TimeAung Phone KhantNo ratings yet
- Financial StressDocument1 pageFinancial StressAung Phone KhantNo ratings yet
- Calendar MakingDocument1 pageCalendar MakingAung Phone KhantNo ratings yet
- f4 StructureDocument39 pagesf4 StructureM Ξ R Λ NNo ratings yet
- Thermal Stability of Hydrogen HalidesDocument81 pagesThermal Stability of Hydrogen HalidesManjesh SharmaNo ratings yet
- Stoichemtry Unit Test Answer KeyDocument11 pagesStoichemtry Unit Test Answer KeyAnred CabahugNo ratings yet
- Determination of Solubility ClassDocument16 pagesDetermination of Solubility ClassAlyssa Alexis RamosNo ratings yet
- Nof0849384523 ch1Document90 pagesNof0849384523 ch1sujit_sekharNo ratings yet
- General Chemistry Topics (1 - 37) : Atomic StructureDocument9 pagesGeneral Chemistry Topics (1 - 37) : Atomic StructureHael CañeteNo ratings yet
- Classification of Solvents: Polar, Nonpolar, Oxygenated & MoreDocument3 pagesClassification of Solvents: Polar, Nonpolar, Oxygenated & MoreOlga BordianNo ratings yet
- Sample Paper Laboratory AnalystDocument11 pagesSample Paper Laboratory AnalystM HUSSAINNo ratings yet
- 11.1 Sugar Luff Schoorl MethodDocument4 pages11.1 Sugar Luff Schoorl MethodYudi Permana100% (2)
- C6 Electrolysis QuestionsDocument19 pagesC6 Electrolysis QuestionsParam BhimaniNo ratings yet
- 8.1 Multiple-Choice and Bimodal QuestionsDocument14 pages8.1 Multiple-Choice and Bimodal QuestionsLong NguyenNo ratings yet
- Fjmnvfuvnfnv Den Cijfb JdncefjnfDocument4 pagesFjmnvfuvnfnv Den Cijfb JdncefjnfHari NirmalNo ratings yet
- CBSE Class 10 Chemistry Worksheet - Chemical Reactions and Equations PDFDocument4 pagesCBSE Class 10 Chemistry Worksheet - Chemical Reactions and Equations PDFMalancha high school HS0% (1)
- Colour Coding of Compressed Gases en 1089-3Document6 pagesColour Coding of Compressed Gases en 1089-3micaziv4786100% (1)
- Calcium chloride: a versatile mineral saltDocument14 pagesCalcium chloride: a versatile mineral saltmdkadryNo ratings yet
- ppsDAFTAR ELEKTROLIT KONSENTRATDocument1 pageppsDAFTAR ELEKTROLIT KONSENTRATNitaNo ratings yet
- Sulphuric Acid - Specification: Indian StandardDocument20 pagesSulphuric Acid - Specification: Indian Standard8085roNo ratings yet
- Formation of Light and Heavy Elements: Prepared By: Jerome A. Bigael, Leyte Progressive High SchoolDocument17 pagesFormation of Light and Heavy Elements: Prepared By: Jerome A. Bigael, Leyte Progressive High Schoolshermaine geniston100% (1)
- AcidBase Equilibrium Ch101EEEDocument343 pagesAcidBase Equilibrium Ch101EEEA. F. M. Mahfuzul KabirNo ratings yet
- ST Thomas Term 1 2016Document16 pagesST Thomas Term 1 2016GunYeij GoodNo ratings yet
- Chlorine, Free and Total, High RangeDocument6 pagesChlorine, Free and Total, High RangeJanette Pinto ApolinarioNo ratings yet
- Metals and Alloys Multiple Choice QuestionsDocument13 pagesMetals and Alloys Multiple Choice Questionsjennifer lohNo ratings yet
- Hsslive Xii Chemistry Chapter 8 SajeevDocument1 pageHsslive Xii Chemistry Chapter 8 SajeevSigma MaleNo ratings yet
- Formation of Elements Big Bang NucleosynthesisDocument3 pagesFormation of Elements Big Bang NucleosynthesisJoseah Mae SaenzNo ratings yet
- Year 7 C-D Chemistry Term 1Document37 pagesYear 7 C-D Chemistry Term 1H ChowdreyNo ratings yet
- List of Ores & AlloyDocument3 pagesList of Ores & AlloySACHINNo ratings yet
- Scheme of Salt AnalysisDocument6 pagesScheme of Salt AnalysisAntony KonikaraNo ratings yet
- Revisionworksheet D22-Jun-2023Document8 pagesRevisionworksheet D22-Jun-2023Prit mistryNo ratings yet
- Chem 16 Sample Problems 1Document1 pageChem 16 Sample Problems 1HeyowJeiNo ratings yet
- Elementary Electrical Engineering - MODULE 2 - What Is ELECTRICITY Is All AboutDocument9 pagesElementary Electrical Engineering - MODULE 2 - What Is ELECTRICITY Is All AboutRubdubRNo ratings yet