Professional Documents
Culture Documents
Female Infertility
Uploaded by
Kiprotich Titus Ngetich0 ratings0% found this document useful (0 votes)
2 views33 pagesCopyright
© © All Rights Reserved
Available Formats
PPTX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
2 views33 pagesFemale Infertility
Uploaded by
Kiprotich Titus NgetichCopyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
You are on page 1of 33
Female Infertility
Definition of terms
• Fecundability: probability of achieving a
pregnancy within 1 menstrual cycle (25%)
• Fecundity: the ability to achieve a live birth
within 1 menstrual cycle (6%)
Evaluation of Female factors
• We will evaluate causes under the following
female factors;
1. Ovulatory Factors
2. The Pelvic Factors
3. Cervical Factors
1. Ovulatory Factors
• Ovulatory dysfunction is responsible for
approximately 20-25% of infertility cases
• Any deviation in the menstrual cycle could
lead to infertility
• Below are some of the causes that affect the
ovulation;
a) Central Defects;
• Chronic hyperandrogenic anovulation
• Hyperprolactinemia – impairs GnRH secretion
• Hypothalamic insufficiency
• Pituitary insufficiency
• Hypothalamic-pituitary dysfunction
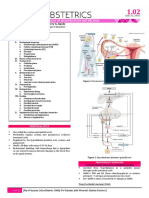
Hypothalamic-Pituitary-Gonadal Axis
b) Peripheral Defects;
• Gonadal dysgenesis (Turner syndrome) –
abnormal development of ovary
• Polycstic ovarian syndrome – hormonal
imbalance, ireegular periods, excess androgen
levels
• Premature ovarian failure
• Ovarian tumor
• Ovarian resistance
c) Metabolic Diseases;
• Thyroid disease – hyperprolactinemia, anovulation
• Liver disease – metabolism of estrogen is impaired,
hence high circulating estrogen
• Renal disease – impairs hypothalamic-pituitary-gonadal
axis
• Obesity – high chances of menstrual disorders and
anovulation
• Androgen excess – affects follicular development hence
anovulation
• Too much exercise – suppress hormones responsible for
ovulation
• Others e.g. drugs – certain antidepressants, medications
with estrogen or progestin
2. Pelvic Factor
• This includes abnormalities of the uterus,
fallopian tubes, ovaries and adjacent pelvic
structures
• They can be ruled out with patient’s history
and investigations
• The causes can include the following;
a) Infection;
• Pelvic inflammatory disease – causes scarring
of fallopian tubes
• STI’s – chlamydia and gonorrhea can damage
uterus, ovaries and fallopian tubes
• Uterine adhesions (Asherman’s syndrome) –
• Appendicitis (ruptured appendix)
b) Endometriosis
• Distorts anatomy of pelvis, adhesions, scarred
fallopian tubes, pelvic structure inflammations,
ovarian problems hence affect ovulation
c) Structural abnormalities
• Failure of normal fusion of the reproductive
tract
• Myoma ( fibroids) – may block fallopian tubes,
or stops a fertilized egg from implanting in the
uterus
• History of ectopic pregnancy
• Lower abdominal surgeries leading to pelvic
adhesions
• Intrauterine polyps
3. Cervical factor
• Abnormalities in the cervix can cause infertility
• Cervical mucus is normally stimulated to change
from thick to thin and stretchable to allow entry of
sperms
• Abnormal cervical mucus may:
– Remain impenetrable to sperm around the time of
ovulation
– Promote sperm destruction by facilitating influx of
vaginal bacteria (e.g. due to cervicitis)
– Contain antibodies to sperm (rarely)
• Mullerian duct abnormality – hence no
development of cervix, uterus, fallopian tubes
• Surgical treatment
• Infections e.g. cervicitis may cause cervical
problems
Diagnosis of infertility in females
• The goal of evaluation is to determine the
causative factor, to provide accurate
information regarding prognosis, to provide
counseling, support and education throughout
the process of evaluation and to provide
guidance regarding options for treatment
• Female infertility evaluation is done based on
the individual factors required for successful
reproduction, i.e. ovulatory, pelvic and cervical
1. Evaluation of Ovulatory factors
• Detailed history should be taken f
• To include; onset of menarche, present cycle
length, presence of premenstrual syndromes
conditions like amenorrhea, oligomenorrhea,
abnormal uterine bleeding
• Signs and symptoms of systemic disease (thyroid
problems) and endocrine disease should be noted
• Also the degree and intensity of exercise, history
of weight loss and complaints of hot flushes
Investigations;
• Hormonal tests – oestrogen, progesterone,
FSH and LH
• Basal body temperature monitoring
• Ultrasonography – to monitor increase in
ovarian follicle diameter, PCOS
2. Evaluation of pelvic factors
• History – to include history of ectopic pregnancy, lower abdominal
surgeries, STI’s, appendicitis, endometriosis, PID
• Tests for STI’s – gonorrhea and chlamydia
• Saline infusion sonohysterography (SIS) – injection of isotonic fluid
through the cervix into the uterus during ultrasonography
• Hysterosalpingography (HSG) – fluoroscopic imaging of the uterus and
fallopian tubes after injection of radiopaque agent into the uterus
• Both of the above are done 2 to 5 days after cessation of menstrual
flow
• Hysteroscopy
• MRI – to rule out endometriosis
• Laparascopy (rare)
3. Evaluation of cervical factors
• History
• Pelvic examination to check for cervicitis and
cervical stenosis
• If cervicitis is suspected, cervical swab taken
to test for gonorrhea or chlamydia
• Complete cervical stenosis is diagnosed if a 1-
2 mm diameter probe cannot be passed into
the uterine cavity
Management
• Management is based on the findings of the
causative factors
• Medical management may include;
– Hormonal medications in cases of ovulatory factors or
endometriosis
– Letrozole – induce ovulation
– Clomiphene - induce ovulation
– Metformin – for those with PCOS, induces ovulation
– Gonadotropins – containing FSH and LH, hCG
– Antibiotics if cervicitis or PID is present
• Another form of management might be
cervical dilation incase of cervical stenosis
• Tubal surgery can also be done in younger
women
Assisted Reproductive Technologies
• They involve manipulation of sperm and ova or embryos in
vitro with the goal of producing a pregnancy
• Oocytes and sperm are collected from the intended parents
or donors, and an embryo or the gametes are transferred to
the woman’s reproductive tract after culture in vitro
• If the risk of genetic defects is high, the embryo can often
be tested for defects before transfer and implantation –
preimplantation genetic testing
• The common methods are intra uterine insemination, in
vitro fertilization (IVF) and intracystoplasmic sperm
injection (ICSI)
Intrauterine insemination (IUI)
• Intrauterine insemination (IUI), also known as
artificial insemination, involves inserting
sperm into the womb via a thin plastic tube
passed through the cervix.
• Sperm is first collected and washed in a fluid.
The best quality specimens (the fastest
moving) are selected.
Intra Uterine Insemination
In Vitro Fertilization (IVF)
• Used to treat infertility due to oligospermia, sperm
antibodies, tubal dysfunction or endometriosis and
also unexplained infertility
• The procedure is as follows;
1. Controlled ovarian stimulation
• A gonadotropin-releasing hormone agonist or
antagonist is given to premature ovulation.
• When the follicular growth is sufficient, human
chorionic gonadotropin (hCG) is given to trigger
follicular maturation and ovulation
2. Oocyte retrieval
• About 34 hours after hCG is given, oocytes are
retrieved by direct needle puncture of the
follicle, usually transvaginally with ultrasound
guidance or less commonly laparoscopically.
• Natural cycle IVF (in which a single oocyte is
retrieved) can be offered as an alternative;
pregnancy rates with this technique are lower
than those with retrieval of multiple oocytes,
but costs are lower and success rates are
increasing.
3. Fertilization:
• The oocytes are inseminated in vitro.
• The semen sample is typically washed several
times with tissue culture medium and is
concentrated for motile sperm, which are then
added to the medium containing the oocytes.
• At this point, intracytoplasmic sperm injection
—injection of a single sperm into each oocyte
—may be done, particularly if
spermatogenesis is abnormal in the male
partner.
4. Embryo culture:
• After sperm are added, the oocytes are
cultured for about 2 to 5 days.
5. Embryo transfer:
• Only 1 or a few of the resulting embryos are transferred
to the uterine cavity, minimizing the chance of a
multifetal pregnancy, the greatest risk of IVF.
• The number of embryos transferred is determined by the
woman’s age and likelihood of response to IVF.
• Some or all embryos (especially if women are at high risk
of ovarian hyperstimulation syndrome) may be frozen in
liquid nitrogen for transfer in a subsequent cycle.
• There is an increasing tendency to place only 1 embryo at
each transfer and to freeze the remaining embryos for
use in subsequent cycles if pregnancy does not result.
Intracytoplasmic sperm injection (ICSI)
• ICSI is useful when other technologies are unsuccessful
or are likely to be so or when a severe sperm disorder
is present.
• Oocytes are obtained as for IVF.
• A single sperm is injected into each oocyte to avoid
fertilization by abnormal sperm.
• The embryo is then cultured and transferred as for IVF.
• There is no benefit to using intracytoplasmic sperm
injection in couples with low oocyte yield or advanced
maternal age.
• If a couple's infertility involves the woman, more
than 30 of these procedures must be done to
make one additional pregnancy likely.
• Thus, the additional costs and risks of
intracytoplasmic sperm injection must be
considered when deciding whether to use it.
• Risk of birth defects may be increased after ICSI,
possibly because of the following:
– The procedure itself can damage the sperm, egg, or
embryo.
– Sperm from men who have mutations of the Y
chromosome may be used. Most reported birth
defects involve the male reproductive tract
Other technologies
• They include the following:
– Use of donor oocytes or embryos
– Transfer of frozen embryos to a gestational carrier
(surrogacy)
Prevention of infertility
• Class discussion
• Thank You!!!
You might also like
- Assisted ReproductionDocument22 pagesAssisted ReproductionAbong Che InnocentNo ratings yet
- Assisted Reproductive Technology (ART) : and ApplicationDocument38 pagesAssisted Reproductive Technology (ART) : and ApplicationPraluki HerliawanNo ratings yet
- Infertility - Female 2021Document51 pagesInfertility - Female 2021Nikky SilvestreNo ratings yet
- 4 Family Having Difficulty Conceiving A ChildDocument10 pages4 Family Having Difficulty Conceiving A ChildDrex CuritanaNo ratings yet
- INFERTILITYDocument67 pagesINFERTILITYIsaacNo ratings yet
- Assisted Reproductive Technology by Rosemary C. AgboDocument19 pagesAssisted Reproductive Technology by Rosemary C. AgboRosemaryNo ratings yet
- Recent Advancement in Infertility ManagementDocument18 pagesRecent Advancement in Infertility ManagementFarheen khan100% (2)
- FertilizationDocument108 pagesFertilizationDauda RukaiyaNo ratings yet
- Recent Advancements in Infertility Techniques AloneDocument111 pagesRecent Advancements in Infertility Techniques AlonesanthanalakshmiNo ratings yet
- Semi Finals CHAPTER IVDocument5 pagesSemi Finals CHAPTER IVjacobprince0016No ratings yet
- Female Infertility: DR Jaqueline Sudiman, PHDDocument21 pagesFemale Infertility: DR Jaqueline Sudiman, PHDGlorio Absalom PuaNo ratings yet
- INFERTILITYDocument19 pagesINFERTILITYAnu LijuNo ratings yet
- 5 Tata Laksana Faktor Uterus - DR DR Sri Ratna - 231021 - 113036Document30 pages5 Tata Laksana Faktor Uterus - DR DR Sri Ratna - 231021 - 113036Edi SabaraNo ratings yet
- 4.. ReproDocument61 pages4.. Reprosabin luitelNo ratings yet
- Female Infertility: DR Jaqueline Sudiman, PHDDocument21 pagesFemale Infertility: DR Jaqueline Sudiman, PHDJuli FionaNo ratings yet
- Abortion: Mohamed Sinan Govt Medical College, CalicutDocument60 pagesAbortion: Mohamed Sinan Govt Medical College, CalicutMhr AliNo ratings yet
- In VitrofertilizationDocument29 pagesIn VitrofertilizationRad RYNo ratings yet
- A Case Presentation: Ectopic PregnancyDocument10 pagesA Case Presentation: Ectopic PregnancyJoshua VillarbaNo ratings yet
- Treatment of InfertilityDocument22 pagesTreatment of InfertilityAlina ShahNo ratings yet
- Infertility 2023Document5 pagesInfertility 2023Charlette GuadalupeNo ratings yet
- Abortion: Mohamed Sinan Govt Medical College, CalicutDocument60 pagesAbortion: Mohamed Sinan Govt Medical College, CalicutYash SharmaNo ratings yet
- Lecture 19infertilityDocument34 pagesLecture 19infertilitySulaiman SanusiNo ratings yet
- Intrauterine Insemination (IUI)Document21 pagesIntrauterine Insemination (IUI)Chanta MaharjanNo ratings yet
- Assisted Reproductive TechnologyDocument14 pagesAssisted Reproductive Technologyarchana jainNo ratings yet
- Ectopic Pregancy: Chena B. CurigDocument35 pagesEctopic Pregancy: Chena B. CurigJaicca Faith Tandih AllasNo ratings yet
- Infertility: DR Madhusudhan C Associate Professor Department of Gen - MedicineDocument20 pagesInfertility: DR Madhusudhan C Associate Professor Department of Gen - MedicineTeena ChandranNo ratings yet
- Infertility: Presented ByDocument47 pagesInfertility: Presented ByNilakshi Barik MandalNo ratings yet
- Ectopic Pregnancy - PowerpointDocument60 pagesEctopic Pregnancy - PowerpointAndrada Doţa100% (1)
- Abortion Sept2021Document51 pagesAbortion Sept2021vrunda joshiNo ratings yet
- Assited Reproductive TechnologyDocument40 pagesAssited Reproductive TechnologySanthosh.S.UNo ratings yet
- Assisted Reproductive TechnologyDocument16 pagesAssisted Reproductive TechnologyQilaNo ratings yet
- Ovarian Cysts: Presented by Neha Barari Assistant Professor SNSRDocument52 pagesOvarian Cysts: Presented by Neha Barari Assistant Professor SNSRBhawna JoshiNo ratings yet
- Understanding Pregnancy and ParenthoodDocument36 pagesUnderstanding Pregnancy and ParenthoodAjay Kumar AtluriNo ratings yet
- Infertility Report...Document9 pagesInfertility Report...sufian JaganyNo ratings yet
- Reproductive HealthDocument80 pagesReproductive HealthSreejithKumarNo ratings yet
- Prepared By: Aashma Bidari M.Sc. Nursing, 1 Year KusmsDocument68 pagesPrepared By: Aashma Bidari M.Sc. Nursing, 1 Year KusmsAasma BidariNo ratings yet
- What Is in Vitro Fertilization (IVF) and Its ProcessDocument4 pagesWhat Is in Vitro Fertilization (IVF) and Its ProcessPlanet womenNo ratings yet
- Infertility: M Onisha Maniappan Ist Year MSC.N KVM ConDocument40 pagesInfertility: M Onisha Maniappan Ist Year MSC.N KVM ConDeepa SudheeshNo ratings yet
- Assisted Reproductive TechnologyDocument12 pagesAssisted Reproductive Technologyvpnayak777No ratings yet
- Assisted Reproductive Technology945Document35 pagesAssisted Reproductive Technology945Praluki HerliawanNo ratings yet
- Infertility, ART, and The Reproductive SystemDocument5 pagesInfertility, ART, and The Reproductive Systemsomeone4630No ratings yet
- 13 InfertilityDocument34 pages13 InfertilityWaad AlNo ratings yet
- Reproductive HealthDocument16 pagesReproductive HealthShazia Khatoon100% (1)
- InfertilityDocument41 pagesInfertilityKiran RoyNo ratings yet
- Infertility: Chegolihina L.VDocument29 pagesInfertility: Chegolihina L.VAmrutesh KhodkeNo ratings yet
- ARTDocument36 pagesARTTtyyfff Fyyu89-No ratings yet
- Infertility Lecture FinalDocument84 pagesInfertility Lecture FinalMohamed Atef Mohamed100% (1)
- 1 MiscarriageDocument34 pages1 Miscarriagezxcvbzaki123No ratings yet
- Chapter 8: Nursing Care of The Subfertile CoupleDocument10 pagesChapter 8: Nursing Care of The Subfertile CoupleAlyssaGrandeMontimorNo ratings yet
- Female Sub FertilityDocument75 pagesFemale Sub FertilityIshaThapaNo ratings yet
- Infertility, Investigation and Management: Dr. Raedah Al-FadhliDocument64 pagesInfertility, Investigation and Management: Dr. Raedah Al-Fadhliapi-3703352No ratings yet
- Recurrent Miscarriage - Pregnancy LossDocument34 pagesRecurrent Miscarriage - Pregnancy Losszianab aliNo ratings yet
- Men and Women's Problems: Jessie Yeo Thian Fang MingDocument6 pagesMen and Women's Problems: Jessie Yeo Thian Fang MingJessiee YeoNo ratings yet
- Reproduction, Technology, and The SocietyDocument38 pagesReproduction, Technology, and The SocietyJep LorenzoNo ratings yet
- INFERTILITYDocument48 pagesINFERTILITYAyisha EdwinNo ratings yet
- Primary Infertility: Zarul Naim Mohd TamiziDocument39 pagesPrimary Infertility: Zarul Naim Mohd TamiziZarul Naim Mohd TamiziNo ratings yet
- Group 2: Presenters Isikanda Mataa Beauty MuntangaDocument76 pagesGroup 2: Presenters Isikanda Mataa Beauty MuntangaShemo MukelabaiNo ratings yet
- Infertility NotesDocument41 pagesInfertility NotesPrasadNo ratings yet
- Female Infertility: Harsha Joseph M17LS25 Msc. Life ScienceDocument10 pagesFemale Infertility: Harsha Joseph M17LS25 Msc. Life ScienceHarsha JosephNo ratings yet
- Breech 2Document67 pagesBreech 2Kiprotich Titus NgetichNo ratings yet
- Abnormal Labour: Mal Presentation and MalpositionDocument41 pagesAbnormal Labour: Mal Presentation and MalpositionKiprotich Titus NgetichNo ratings yet
- Infertility in Males and FemalesDocument18 pagesInfertility in Males and FemalesKiprotich Titus NgetichNo ratings yet
- MenopauseDocument15 pagesMenopauseKiprotich Titus NgetichNo ratings yet
- Advanced Sexual Practices Volume1Document252 pagesAdvanced Sexual Practices Volume1sumeetakumar62% (21)
- Abnormal Uterine BleedingDocument11 pagesAbnormal Uterine BleedingKIPA SHRESTHANo ratings yet
- The Sexual: SEL FDocument81 pagesThe Sexual: SEL FClarice LangitNo ratings yet
- 'Learner's Activity Sheet Assessment Checklist: Third Quarter - Week 2Document12 pages'Learner's Activity Sheet Assessment Checklist: Third Quarter - Week 2Kareena Ameenah BasmanNo ratings yet
- Menstrual Cycle Alters Face PreferenceDocument2 pagesMenstrual Cycle Alters Face PreferenceInterestingScienceNo ratings yet
- Science 10 - Activity 2 - 3rd Quarter - MontereyDocument2 pagesScience 10 - Activity 2 - 3rd Quarter - MontereyKiel Angelo MontereyNo ratings yet
- Can Hormonal Birth Control Affect Your Ability To Pick Up On Social CuesDocument5 pagesCan Hormonal Birth Control Affect Your Ability To Pick Up On Social CuespkmgontaNo ratings yet
- ST 2 Gr. 5 Science With TosDocument4 pagesST 2 Gr. 5 Science With TosEG Bitong-AlamaniNo ratings yet
- OB 1 - 1.02 Physiology of Menstruation and Decidua PDFDocument11 pagesOB 1 - 1.02 Physiology of Menstruation and Decidua PDFMDreamerNo ratings yet
- General Biology 2 NotesDocument17 pagesGeneral Biology 2 NotesAlyssa Mae BinoNo ratings yet
- Unit 6 - : Reproductive System Test BankDocument10 pagesUnit 6 - : Reproductive System Test Bankjayrald cruzada100% (1)
- The Electrical Patterns of Life - The Work of Dr. Harold SDocument4 pagesThe Electrical Patterns of Life - The Work of Dr. Harold SSilvio ArévalosNo ratings yet
- Mapeh7-1st Quarter ExamDocument8 pagesMapeh7-1st Quarter ExamViolet DV BalinoNo ratings yet
- Oestrus CycleDocument20 pagesOestrus CycleHassim M H SNo ratings yet
- X-IGCSE Half Yearly 2023Document20 pagesX-IGCSE Half Yearly 2023Dr. M. PrinceNo ratings yet
- Anatomy Exam 100 MARKS 04 (1) .07-1Document13 pagesAnatomy Exam 100 MARKS 04 (1) .07-1aishaalthaf.4.4No ratings yet
- Menstrual CycleDocument14 pagesMenstrual CycleVáncsa Szilárd100% (2)
- Primolut N NorethisteroneDocument2 pagesPrimolut N NorethisteroneSarathyTDNo ratings yet
- DLL Q3 Week 4.reprouctiveDocument4 pagesDLL Q3 Week 4.reprouctivekaycin DuzonNo ratings yet
- Topic 7 Diagnostic Cytology (Telegram Discussion)Document6 pagesTopic 7 Diagnostic Cytology (Telegram Discussion)Laiza Fathma AbilosaNo ratings yet
- SOAL Masuk 2018Document142 pagesSOAL Masuk 2018Yoza FirdaozNo ratings yet
- Pathology Handbook - Royal Cornwall Hospital Trust 2011Document136 pagesPathology Handbook - Royal Cornwall Hospital Trust 2011Fuzzy_Wood_PersonNo ratings yet
- Ge 11 Prefinals Human Reproduction Another HandoutDocument9 pagesGe 11 Prefinals Human Reproduction Another HandoutotakukairoNo ratings yet
- Yearly Lesson Plan KSSM Science DLP Form 1 2021Document40 pagesYearly Lesson Plan KSSM Science DLP Form 1 2021nur afiqahNo ratings yet
- Endometrial BiopsyDocument72 pagesEndometrial BiopsySatish TatamiyaNo ratings yet
- Yahoo Site Admin Assets Docs Genetics Practice Examination 2.266183007Document11 pagesYahoo Site Admin Assets Docs Genetics Practice Examination 2.266183007Steven Breus100% (1)
- Coordinated Functions of The Nervous, Endocrine, and Reproductive SystemsDocument30 pagesCoordinated Functions of The Nervous, Endocrine, and Reproductive SystemsSarah CruzNo ratings yet
- OB Definition of TermsDocument9 pagesOB Definition of TermsWarrenSandovalNo ratings yet
- Gynecology 2.01.2 Benign Neoplasms of The Ovary and Oviducts - Dr. Co-HidalgoDocument7 pagesGynecology 2.01.2 Benign Neoplasms of The Ovary and Oviducts - Dr. Co-HidalgoMarc Francis AlayNo ratings yet
- Dysmenorrhoea PDFDocument7 pagesDysmenorrhoea PDFRafiqy Sa'adiy FaizunNo ratings yet