Professional Documents
Culture Documents
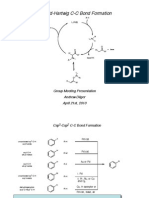
12 Suzuki
12 Suzuki
Uploaded by
gfshrhrCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
12 Suzuki
12 Suzuki
Uploaded by
gfshrhrCopyright:
Available Formats
Myers
Reviews:
The Suzuki Reaction
Analysis of Elementary Steps in the Reaction Mechanism
Chem 215
Suzuki, A. In Metal-Catalyzed Cross-Coupling Reactions, Diederich, F., and Stang, P. J., Eds.; Wiley-VCH: New York, 1998, pp. 49-97. Miyaura, N.; Suzuki, A. Chem. Rev. 1995, 95, 24572483. Suzuki, A. J. Organometallic Chem. 1999, 576, 147168.
B-Alkyl Suzuki reaction:
Chemler, S. R.; Trauner, D.; Danishefsky, S. J. Angew. Chem., Int. Ed. Engl. 2001, 40, 45444568.
Oxidative Addition
Br Pd0Ln Br Pd L isomerization L Pd Br L trans
II
L cis Relative reactivity of leaving groups: I > OTf > Br >> Cl .
Solid phase: Franzn, R. Can. J. Chem. 2000, 78, 957962.
Oxidative addition is known to proceed with retention of stereochemistry with vinyl halides and with inversion with allylic and benzylic halides. Stille, J. K.; Lau, K. S. Y. Acc. Chem. Res. 1977, 10, 434442. Oxidative addition intially gives a cis complex that rapidly isomerizes to its trans isomer.
The Suzuki reaction is the coupling of an aryl or vinyl boronic acid with an aryl or vinyl halide or triflate using a palladium catalyst. It is a powerful cross coupling method that allows for the synthesis of conjugated olefins, styrenes, and biphenyls:
Casado, A. L.; Espinet, P. Organometallics 1998, 17, 954959.
Transmetallation
L Pd Br
II
n-Bu
B O O
benzene/NaOEt Br 80 C, 4 h 98%
n-Bu
+
NaOEt
Et O PhL2Pd B
O O
L Pd L
II
n-Bu
+ O
n-Bu
Miyaura, N.; Suzuki, A. J. Chem. Soc., Chem. Commun. 1979, 866867. Mechanism:
B O O
n-Bu
EtO B
O
Organoboron compounds are highly covalent in character, and do not undergo transmetallation readily in the absence of base. The role of the base during transmetalation is unresolved. Boron "ate" complexes, formed via quaternization of the boron with a negatively charged base, are frequently invoked.
n-Bu
Pd0Ln
Ph Br Oxidative Addition
Reductive Elimination Ph Ph Br
Matos, K.; Soderquist, J. A. J. Org. Chem. 1998, 63, 461470.
Reductive Elimination n-Bu
EtO B O O
PdIILn PdIILn
L NaOEt Pd L trans
II
n-Bu
L cis
L Pd
II
n-Bu
n-Bu +
Pd0Ln
Ph
n-Bu
B O O
EtO
PdIILn
NaBr
Isomerization to the cis complex is required before reductive elimination can occur.
Transmetallation
Relative rates of reductive elimination from palladium(II) complexes: arylaryl > alkylaryl > n-propyln-propyl > ethylethyl Miyaura, N.; Suzuki, A. Chem. Rev. 1995, 95, 2457-2483.
>
methylmethyl
Suzuki, A. Pure & Appl. Chem. 1985, 57, 17491758.
Andrew Haidle/Chris Coletta
Conditions R BY2 + X R' P(PPh3)4 benzene, 80 C base time (h) 2 R R'
Catalyst and Ligands: The most commonly used system is Pd(PPh3)4, but other palladium sources have been used including PdII pre-catalysts that are reduced to the active Pd0 in situ:
- Pd2(dba)3 + PPh3 - Pd(OAc)2 + PPh3 - PdCl2(dppf) (for sp3-sp2 couplings-see section on B-alkyl Suzuki reaction)
yield (%) 80a
n-Bu
B O O
Br Ph
Ph
NaOEt
"Ligand-free" conditions, using Pd(OAc)2, have also been developed. Side reactions often associated with the use of phosphine ligands (phosphonium salt formation and aryl-aryl exchange between substrate and phosphine) are thus avoided. Goodson, F. E.; Wallow, T. I.; Novak, B. M. Org. Synth. 1997, 75, 6168. Use of N-heterocyclic carbenes as an alternative to phosphine ligands:
CH3 H3C N N CH3 H3C CH3 N + N Cl CH3 H3C 2
Pd2(dba)3 (1.5 mol%), 2 (3 mol%), Cs2CO3
NaOEt Br Br CH3 CH3 I Ph Br Ph NaOEt NaOEt NaOEt NaOEt
80a
H3C
NaOEt
2 2 2 4 2
81a 100b 63b 98b 3b
H3C
CH3 H3C 1 + Cl (HO)2B
CH3
H3C
dioxane, 80 C, 1.5h 96%
H3C
Cl Ph H3CO Br
The nucleophilic N-heterocyclic carbene 1 is the active ligand, and is formed in situ from 2. The use of ligand 1 allows for utilization of aryl chlorides in the Suzuki reaction (see the section on bulky, electron rich phosphines as ligands for use of aryl chlorides as coupling partners as well).
NaOEt
93b
Zhang, C.; Huang, J.; Trudell, M. L.; Nolan, S. P. J. Org. Chem. 1999, 64, 3804-3805.
B(OH)2
I Ph Br Ph Cl Ph Br
2M NaOH 2M Na2CO3 NaOEt 2M NaOH
6 6 6 2
62c 88c 0c 87d
Organoboranes: A variety of organoboranes may be used to effect the transfer of the organic coupling partner to the reactive palladium center via transmetallation. Choice of the appropriate organoborane will depend upon the compatability with the coupling partners and availability (see section on synthesis of organoboranes). Some of the more common organoboranes used in the Suzuki reaction are shown below:
O R B(OH)2 R B R B(OiPr)2 R B R B O R B O O CH3 CH3 CH3 CH3
n-Bu
B Br 2M NaOH 2 99d
Use of Aryltrifluoroborates as Organoboranes for the Suzuki Reaction:
The conditions shown above are the original conditions developed for the cross coupling by Suzuki and Miyaura. The reaction is stereo- and regiospecific, providing a convenient method for the synthesis of conjugated alkadienes, arylated alkenes, and biaryls. Note that under the conditions shown above, aryl chlorides are not acceptable substrates for the reaction, likely due to their reluctance to participate in oxidative addition.
a Miyaura, b Miyaura, c Miyaura, d Miyaura,
OCH3 CH3OH, reflux 2h, 95% The aryltrifluoroborates are prepared by treatment of the corresponding arylboronic acid with excess KHF2. According to the authors, aryltrifluoroborates are more robust, more easily purified, and less prone to protodeboronation compared to aryl boronic acids. Molander, G. A.; Biolatto, B. J. Org. Chem. 2003, 68, 43024314.
BF3K
Br
OCH3
Pd(OAc)2, K2CO3
N.; Yamada, K.; Suzuki, A. Tetrahedron Lett. 1979, 20, 34373440. N.; Suzuki, A. J. Chem. Soc., Chem. Commun. 1979, 866867. N.; Yanagi, T.; Suzuki, A. Synth. Commun. 1981, 11, 513519.
Solvent: The Suzuki reaction is unique among metal-catalyzed cross-coupling reactions in that it can be run in biphasic (organic/aqueous) or aqueous environments in addition to organic solvents. Casalnuovo, A. L.; Calabrese, J. C. J. Am. Chem. Soc. 1990, 112, 43244330.
N.; Yano, T.; Suzuki, A. Tetrahedron Lett. 1980, 21, 28652868.
Andrew Haidle/Chris Coletta
TlOH and TlOEt as Rate-Enhancing Additives for the Suzuki Reaction
TBSO I TBSO OTBS OTBS + OTBS OTBS OTBS O OTBS OCH3 OTBS OTBS
Bulky, Electron-Rich Phosphines as Ligands for the Suzuki Reaction
R R
TBSO (H3C)2N OTBS OTBS OTBS O OTBS OCH3 R R = PCy2 (1) R = P(t-Bu)2 (2) Cl + (HO)2B R CH3 CH3 ligand PPh3 Pt-Bu3 4 Pt-Bu3 4 Pt-Bu3 Pt-Bu3 Pd source Pd2(dba)3 Pd2(dba)3 Pd(OAc)2 Pd2(dba)3 Pd(OAc)2 Pd2(dba)3 Pd2(dba)3 base Cs2CO3 Cs2CO3 KF Cs2CO3 KF Cs2CO3 Cs2CO3 solvent dioxane dioxane THF dioxane THF dioxane temp (C) time (h) yield (%) 80 80 23 80 45 80 5 5 6 5 6 5 0a 86a 95b 89a 93b 92a 91a R = PCy2 (3) R = P(t-Bu)2 (4)
TBSO (HO)2B
base KOH TlOH
temp (C) 23 23
time 2h <<30 sec
yield 86 92
relative rate 1 1000
TlOH greatly increases the rate of coupling, which the authors attribute to acceleration of the hydroxylhalogen exchange at palladium. Uenishi, J.; Beau, J.; Armstrong, R. W.; Kishi, Y. J. Am. Chem. Soc. 1987, 109, 47564658.
CH3O
NH2 O
dioxane
80
TlOH vs. TlOEt
H3C Previous to the introduction of the bulky, electron-rich phosphine ligands shown above, most aryl chlorides were not suitable substrates for the Suzuki reaction.
t-BuO2C CO2t-Bu
(HO)2B (5 equiv)
OH
t-BuO2C CO2t-Bu
These ligands are either commercially available (Pt-Bu3) or readily synthesized from commercial starting materials (1-4). The increased activity of the ligands shown above allows for the Suzuki reaction of aryl bromides at room temperature.
a Littke,
Pd(PPh3)4 (10 mol %) I CO2Me TlOEt (1.8 equiv) 3:1 THF:H2O 97% HO CO2Me
A. F.; Dai, C.; Fu, G. C. J. Am. Chem. Soc. 2000, 122, 40204028.
J. P.; Singer, R. A.; Yang, B. H.; Buchwald, S. L. J. Am. Chem. Soc. 1999, 121, 95509561.
b Wolfe,
THF (anhydrous) 93%
Alkyl Di-tert-butylphosphane-Ligated Palladium(I) dimers as catalyst for the Suzuki Reaction
Roush has found that TlOEt is a generally superior source of Tl for Suzuki couplings. Pure TlOH is available from only a single source, aqueous solutions of TlOH have a poor shelf life, and the aqueous solutions are both air- and light-sensitive. The largest problem with using TlOEt is that some boronic acidTlOEt adducts are not very soluble. Using water as a cosolvent helps to alleviate this problem in many cases.
PdI[P(1-Ad)t-Bu2] Br KOH, THF, 15 min, RT 95% As a solid, the catalytic complex is stable indefinitely in the air. it is believed that the catalyst fragments to form the monomeric subunits under the reaction conditions.
[t-Bu2(1-Ad)P]PdI
Br
Br + (HO)2B
Frank, S. A.; Chen, H.; Kunz, R. K.; Schnaderbeck, M. J.; Roush, W. R. Org. Lett. 2000, 2, 26912694.
Reactions of phenyl boronic acids with (deactivated) aryl chlorides occurred rapidly at room temperature, but conversion did not exceed 70%.
Stambuli, J. P.; Kuwano, R.; Hartwig, J. F. Angew. Chem. Int. Ed. 2002, 41, 4746-4748.
Chris Coletta
B-Alkyl Suzuki Reaction
sp3-sp3 Suzuki Coupling
R' Br
B R
PdCl2(dppf) (3 mol%) THF, NaOH, reflux
R R'
By employing a bulky, electron-rich ligand (similar to the ligands used in aryl chloride Suzuki couplings) Fu and coworkers are able to effect the Suzuki coupling of primary alkyl bromides or chlorides and 9-alkyl-9BBN:
vinyl bromide Br CH3 THPO CH3 Br CH3 O Br
9-alkyl-9-BBN 9-BBN 9-BBN
2
product CH3
6
yield (%) + 85 B Br CH3
9
4% Pd(OAc)2 8% PCy3 K2PO4H2O 85%
Ph
CH3
7
Ph OCH3 OCH3 CH3 THPO CH3
CH3
9
OCH3 80 OCH3 CH3 CH3 O
6
Netherton, M. R.; Dai, C.; Klaus, N.; Fu, G. C. J. Am. Chem. Soc. 2001, 123, 10099-10100.
9-BBN
CH3
7
98
+ Cl B
4
OTBS
5% Pd2(dba)3 20% PCy3 CsOHH2O dioxane, 90 C 72%
OTBS
4
9-BBN CH3 CH3 Br
CN
8
CH3 CH3
81 (CH2)8CN
Kirchhoff, J. H.; Dai, C.; Fu, G. C. Angew. Chem., Int. Ed. Engl. 2002, 41, 1945-1947.
With the advent of the PdCl2(dppf) catalyst, primary alkyl groups can be transferred by Suzuki coupling, typically using 9-BBN reagents.
Other suitable coupling partners include aryl or vinyl triflates and aryl iodides. Secondary alkyl boron compounds are not suitable coupling partners for this reaction.
Suzuki Cross-Coupling of Unactivated Secondary Alkyl Halides
Recently, Fu and coworkers have developed a Ni0-catalyzed Suzuki coupling of unactivated alkyl bromides and iodides:
Alkyl boronic esters are also be viable substrates in the B-alkyl Suzuki reaction when thallium salts such as TlOH or Tl2CO3 are used as the base. Miyaura, N.; Ishiyama, T.; Sasaki, H.; Ishikawa, M.; Satoh, M.; Suzuki, A. J. Am. Chem. Soc. 1989, 111, 314321. Sato, M.; Miyaura, Suzuki, A. Chem. Lett. 1989, 1405-1408. The large bite angle of the dppf ligand has been noted and is believed to provide a catalyst with a more favorable ratio of rate constants for reductive elimination versus -hydride elimination. 88 Pd PPh2 Ph2P 99 PPh2 I + (HO)2B Br + (HO)2B
Ph
Ph
N 1
4% Ni(cod)2 8% 1 KOt-Bu, s-butanol 60 C, 5h 91 %
PPh2 dppf = Fe
Cl
Cl 4% Ni(cod)2 8% 1 O O KOt-Bu, s-butanol 60 C, 5h 62 %
Hayashi, T.; Konishi, M.; Kobori, Y.; Kumada, M.; Higuchi, T.; Hirotsu, K. J. Am. Chem. Soc. 1984, 106, 158163. Brown, J. M.; Guiry, P. J. Inorg. Chim. Acta. 1994, 220, 249259.
Zhou, J.; Fu, G. C. J. Am. Chem. Soc. 2004, 126, 1340-1341.
Chris Coletta
(+)-Discodermolide (B-Alkyl Suzuki Reaction)
Rutamycin B
I Ph3P CH3
CH3 CH3 O O TBSO MOMO PMP CH3
tBuO
CH3 CH3 OTBS O O H CH3 CH3 B(OH)2 I CH3 OTES 1. TlOH (2.6 equiv) 2. B (0.36 equiv) 3. Pd(PPh3)4 (20 mol %) THF, 23 C, 45 min 77% B = CH3
CH3 (2 equiv)
CHO OMOM
78 C 23 C, THF, 2.5 h 40 %
OTBS O OTBS CH3 CH3 CH3
CH3 CH3 O TBSO
CH3 CH3 CH3 I
E:Z
15:85
O TBSO MOMO PMP A CH3 OMOM
1. tBuLi (2 eq) CH3 CH3 CH3 I 2. PMBO OTES THF 1. K3PO4 (2.3 equiv), 23 C 2. A, DMF 3. PdCl2(dppf) (10 mol %), 16 h 74% CH3 CH3 CH3 O O TBSO MOMO PMP CH3 OMOM CH3 OPMB OTES CH3 CH3 CH3 CH3 BOCH3 Et2O, 78 C CH3O B PMBO OTES CH3 CH3 CH3 Li CH3 OTBS CH3 O O H
tBuO
CH3 OTES CH CH3
3
OTBS O OTBS CH3 CH3 CH3
CH3 O
TBSO
CH3 CH3 CH3 HO CH3 O O OH CH3 OH (+)-Discodermolide Marshall, J. A.; Johns, B. A. J. Org. Chem. 1998, 63, 78857892. CH3 OH O CH3 O NH2 CH3 Rutamycin B CH3 CH3 CH3 OH O O H
CH3 O O CH3 CH3 CH3 O HO OH O OH CH3 CH3 CH3
Evans, D. A.; Ng, H. P.; Rieger, D. L. J. Am. Chem. Soc. 1993, 115, 1144611459.
Andrew Haidle
Palytoxin Amide: O TEOCN H
O O OTBS H TBSO O O O OTBS OTBS A CH3 CH3 TBSO CH TBSO
3
OTBS
OTBS 1. (COCl)2, DMSO, Et3N, 78 0 C OTBS O O TBSO TMEDA, THF, 0 C; 2. B B O O OH Li EtOAc; NaCl, HCl
OTBS OTBS TBSO (HO)2B
3. TlOH (10% aq); B, Pd(PPh3)4, 23 C 4. LiCH2P(O)(OCH3)2 (30 eq.)/THF, 78 C 7580%
I OAc OTBS OTBS OTBS OTBS TBSO O TBSO CH3O O B H2N O OH H HO OH OH O H2N OH CH3 O O OH CH3 OH CH3 HO OH O H HO OH OH OH OH CH3 OH O HO HO OH Kishi, Y., et al. J. Am. Chem. Soc. 1989, 111, 75257530. Kishi, Y., et al. J. Am. Chem. Soc. 1989, 111, 75307533. Palytoxin amide OH CH3 OH HO H OH HO O OH OH H OTBS OTBS OTBS O O OH OH O CH OH 3 OH OH OH OH OH HO OH HO HO OH OH O TEOCN H OTBS H TBSO
O O O O O CH3 OTBS OTBS TBSO CH3 CH3TBSO OTBS TBSO OTBS OTBS
OAc OTBS OTBS OTBS OTBS TBSO O TBSO (CH3O)2 P O H OTBS O OTBS OTBS
CH3
OH OH
Andrew Haidle
Epothilone A:
Synthesis of organoboron compounds:
S CH3 N CH3 (CH3)3Si CH3 1. N-iodosuccinimide AgNO3, acetone 0 23 C, 1.5 h CH3 3. PhSH, BF3OEt2 CH2Cl2, 23 C 4. Ac2O, Py, 4-DMAP CH2Cl2, 23 C 35% CH3 S CH3 N CH3 H PdCl2(dppf)2 (10 mol %) OAc Ph3As (10 mol %) CsCO3, H2O, DMF 4 h, 23 C; I Ishiyama, T.; Itoh, Y.; Kitano, T.; Miyaura, N. Tetrahedron Lett. 1997, 38, 34473450. Ishiyama, T.; Murata, M.; Miyaura, N. J. Org. Chem. 1995, 60, 75087510. S CH3 CH3 N CH3 O H O O OH CH3 Epothilone A CH3 O OH CH3 CH3 CH3 S N CH3 H CH3 2 h, 23 C, 75% Aryl bromides and chlorides can also be used. B CH3 CH3 OCH3 OCH3 OTIPS OTBS O2N OTf 2. (c-Hex)2BH Et2O, AcOH, 23 C CH3 CH3 OCH3 OCH3 OTIPS OTBS Brown, H. C.; Cole, T. E. Organometallics 1983, 2, 13161319. Li H R BX2
OMOM
1. B(Oi-Pr)3 (1 equiv) Et2O, 78 C 23 C, 4 h 2. HCl/Et2O, 0 C, 30 min 84% B(Oi-Pr)2
9-BBN THF, 23 C CH3 CH3 CH3 CH3
CH3 CH3 CH3 CH3 O O2N B O CH3 CH3 CH3 CH3
O B O B
O O
PdCl2(dppf) (3 mol %) KOAc, DMSO, 80 C 86%
OAc CH3 CH3 CH3
OCH3 OCH3 OTIPS OTBS
BX2 R
n-Bu
Br
1. HBBr2S(CH3)2 2. i-PrOH 87%
Br
n-Bu
KHB(Oi-Pr)3 n-Bu B(Oi-Pr)2 Et2O
89%
B(Oi-Pr)2
Meng, D.; Bertinato, P.; Balog, A.; Su, D.; Kamenecka, T.; Sorensen, E. J.; Danishefsky, S., J.
J. Am. Chem. Soc. 1997, 119, 1007310092.
Brown, H. C.; Imai, T. Organometallics 1984, 3, 13921395.
Andrew Haidle
1. n-BuLi,
n-Hex
Et2O, 78 C H 2. B(Oi-Pr)3 3. HCl/Et2O 95%
H2
B(Oi-Pr)2
R' R BX2
n-Hex
B(Oi-Pr)2
n-Hex
Lindlar cat. 82% 95:5 E:Z
Brown, H. C.; Bhat, N. G.; Srebnik, M. Tetrahedron Lett. 1988, 29, 26312634. Srebnik, M.; Bhat, N. G.; Brown, H. C. Tetrahedron Lett. 1988, 29, 26352638. [Rh(cod)Cl]2 (1.5 mol %) P(i-Pr)3 (6 mol %) O TBSO (1.2 equiv) Et3N (1.0 equiv) cyclohexane, 20 C, 2 h HB O O (1.0 equiv)
n-Bu
1. (Ipc)2BH 2. CH3CHO
n-Bu
pinacol B(OEt)2
n-Bu
B O
CH3 CH3
CH3 CH3 CH3 HO OH CH3 = pinacol CH3
TBSO B O
n-Bu
CH3 CH3
B O
CH3 CH3
EtZnI PdCl2(dppf) (1 mol%) Et2O, 25 C, 2 h 67%
CH3 CH3
Moriya, T.; Miyaura, N.; Suzuki, A. Chemistry Lett. 1993, 14291432. 72 %, 98 % Z
Ohmura, T.; Yamamoto, Y.; Miyaura, N. J. Am. Chem. Soc. 2000, 122, 49904991.
BX2 R R'
BX2 Br O B O O
n-Hex
O HB O
n-BuLi
Et2O, 78 C 83%
O CH3
n-Hex
n-Pr
H THF, 70 C 80%
n-Pr
B O
O Grignard reagents can also be used. Brown, H. C.; Imai, T.; Bhat, N. G. J. Org. Chem. 1986, 51, 52775282.
Brown, H. C.; Gupta, S. K. J. Am. Chem. Soc. 1972, 94, 43704371.
n-Bu n-Bu
H 1. Et3SiH, 78 C 2. BCl3
Br
n-HexBHBrS(CH3)2
Et2O, 0 C
Br
n-Bu
B Br
n-Hex
NaOCH3 CH3OH
B(OCH3)2
n-Bu
n-Hex
> 75%
n-Bu
HO BCl2 83%
OH
n-Bu
B O
Exact yield not specified because the vinyl borane shown was oxidized to a ketone. Soundararajan, R.; Matteson, D. S. J. Org. Chem. 1990, 55, 22742275. Brown, H. C.; Basavaiah, D.; Kulkarni, S. U. J. Org. Chem. 1982, 47, 38083810.
Andrew Haidle
Comparison of the Stille and Suzuki cross-coupling methods: B(OH)2 The yields are often comparable: OCH3 OCH3 O MenO TfO OCH3 Pd(PPh3)4 (5 mol %) CuI (4 mol %) LiCl (4.4 equiv) BHT (1.8 mol %) OtBu O MenO CH3O NH O OtBu Holzapfel, C. W.; Dwyer, C. Heterocycles 1998, 48, 15131518. CH3 O CH3 NO2 Pd(PPh3)4 (10 mol %) K3PO4 DME, 85 C OCH3 82% OTf NO2 Pd2dba3 (5 mol %) P(o-tol)3 (40 mol %) LiCl, dioxane, 85 C 80% NO2 Sn(CH3)3 OCH3
(CH3)3Sn
H N
pdioxane, reflux, 1 h
81%
CH3O
Some highly sensitive compounds do not tolerate the basic conditions of the Suzuki reaction. Farina, V.; Krishnamurthy, V.; Scott, W. J. Org. React. 1998, 50, 1652.
O MenO TfO
CH3 O OCH3 Pd(PPh3)4 (4 mol %) Na2CO3 MenO CH3O NH O OtBu CH3
When alkylboron and alkylstannane groups are present in the same molecule, the organoboron groups react preferentially under basic conditions. O OCH3 CH3 PdCl2(dppf) (3 mo l%) Br B Sn(CH3)3 K3PO4, DMF 5060 C, 88% O CH3 Sn(CH3)3
pdioxane, reflux, 45 min
(HO)2B H N O OtBu 90%
CH3O
Ishiyama, T.; Miyaura, N.; Suzuki, A. Synlett 1991, 687688. The cross-coupling reaction of primary organoboranes is possible, while primary organo OH O = Men CH3 OH O HO (+)-Dynemicin A H HN CH3 COOH O OCH3 H S S CH3 H 1. 9-BBN, THF, 0 C 2. Br OAc CH3 CH3 CO2CH3 S S CH3 H stannanes are not typically used. CH3O2C CH3
CH3
CH3
PdCl2(dppf) (15 mol %) K2CO3, DMF, 50 C The higher cost and toxicity of organostannanes makes the Suzuki coupling the preferred method. 77% Myers, A. G.; Tom., N. J.; Fraley, M. E.; Cohen, S. B.; Madar, D. J. J. Am. Chem. Soc. 1997, 119, 60726094.
OAc CH3
Uemura, M.; Nishimura, H.; Minami, T.; Hayashi, Y. J. Am. Chem. Soc. 1991, 113, 54025410.
Andrew Haidle
Stille couplings with primary organostannanes typically involve special structural features, such as an -heteroatom, and typically cannot undergo -hydride elimination.
In the following examples, the Suzuki coupling was successful but the corresponding Stille reaction failed. This was attributed to a proposed slower rate of transmetalltion in the Stille reaction. CH3O O CH3O I CH3O O OCH3 O
Bu3Sn Br
OCH3 (1.3 equiv) OCH3
CH3O
H3C CH 3 O CH3 B O CH3 OCH3
PdCl2(PPh3)2 (1 mol %) HMPA, 80 C, 20 h 73%
PdCl2(dppf) (3 mol %) K3PO4, DMF, 5060 C 88%
Kosugi, M.; Sumiya, T.; Ogata, T.; Sano, H.; Migita, T. Chemistry Lett. 1984, 12251226.
OCH3 The Stille coupling has been used for the introduction of glycosylmethyl groups. CH3O O CH3O CH3O H3C O O CH3 H OTBS CH3 O H CH3 CH3 OCH3 OTf Pd(PPh3)4 (20 mol %) LiCl (40 equiv) THF, 130 C 52% CH3 H CH3 O AcO O Bu3Sn O O OAc (7.6 equiv) CH3 CH3 H OTBS CH3 O OCH3 O CH3O O CH3 CH3 CH3O I CH3O O OCH3 O O CH3O O O OCH3 OCH3 O O
versus CH3O Sn(CH3)3 OCH3 various conditions CH3O H CH3O O O
CH3O
Sn(CH3)3 OCH3
Chen, X.-T.; Bhattacharya, S. K.; Zhou, B.; Gutteridge, C. E.; Pettus, T. R. R.; Danishefsky, S. J.
J. Am. Chem. Soc. 1999, 121, 65636579.
O OCH3
Zembower, D.E.; Zhang, H. J. Org. Chem. 1998, 93009305.
Andrew Haidle
You might also like
- HRTEMDocument5 pagesHRTEMRajathi YadavNo ratings yet
- Suzuky ReactionDocument13 pagesSuzuky ReactionAlbornoz JuanNo ratings yet
- Chem 215 Myers: The Heck ReactionDocument8 pagesChem 215 Myers: The Heck ReactiondubstepoNo ratings yet
- Eshon Vangal Cross Coupling ReactionsDocument47 pagesEshon Vangal Cross Coupling ReactionsCadotNo ratings yet
- 9 Lithium HalogenDocument3 pages9 Lithium HalogenAndy ChengNo ratings yet
- Oxidation (Myers)Document10 pagesOxidation (Myers)YouTibeNo ratings yet
- Olefin Metathesis in Organic SynthesisDocument19 pagesOlefin Metathesis in Organic SynthesisaegosmithNo ratings yet
- Piazza ClaudiaDocument160 pagesPiazza Claudiar_lusyy_100% (1)
- 1 s2.0 S0277538710005255 MainDocument9 pages1 s2.0 S0277538710005255 MainMohammad Imran HossainNo ratings yet
- 7 Magnesium HalogenDocument5 pages7 Magnesium HalogenMai ChiNo ratings yet
- AKD BuchwaldhartwigDocument39 pagesAKD BuchwaldhartwigMurali Venkat NagNo ratings yet
- Chem 215 Myers: Birch ReductionDocument7 pagesChem 215 Myers: Birch ReductionPrasanna AndojuNo ratings yet
- Polymer Bound CatalystsDocument12 pagesPolymer Bound CatalystsStefan GherghinaNo ratings yet
- Catalytic C H Functionalization Driven by CO As A Stoichiometric Reductant: Application To Carbazole SynthesisDocument3 pagesCatalytic C H Functionalization Driven by CO As A Stoichiometric Reductant: Application To Carbazole SynthesisJORGE IVAN CASTRO CASTRONo ratings yet
- Iodination Nis-Tfa PDFDocument2 pagesIodination Nis-Tfa PDFBrandon TimmNo ratings yet
- Chemistry 206 Advanced Organic Chemistry: Simmons-Smith Reaction: Enantioselective VariantsDocument0 pagesChemistry 206 Advanced Organic Chemistry: Simmons-Smith Reaction: Enantioselective VariantseraborNo ratings yet
- Hydrozirconation - Final 0Document11 pagesHydrozirconation - Final 0David Tritono Di BallastrossNo ratings yet
- Chem 115 Myers: Birch ReductionDocument7 pagesChem 115 Myers: Birch ReductionNimz02No ratings yet
- 28 CyclopropanationDocument11 pages28 CyclopropanationRajesh TammanaNo ratings yet
- Oxidation Reactions of Organic ChemistryDocument28 pagesOxidation Reactions of Organic ChemistryviejayNo ratings yet
- SE OMC Part1 2023Document54 pagesSE OMC Part1 2023tharun thennarasuNo ratings yet
- Yamamoto Et Al. - 2009 - Γ-Selective Cross-Coupling Reactions of PotassiumDocument9 pagesYamamoto Et Al. - 2009 - Γ-Selective Cross-Coupling Reactions of PotassiumVictor CiocalteaNo ratings yet
- Arkivoc 2017, V, 314-326Document13 pagesArkivoc 2017, V, 314-326RohanNo ratings yet
- Iodine-Catalyzed Aminosulfonation of Hydrocarbons by Imidoiodinanes. A Synthetic and Mechanistic InvestigationDocument7 pagesIodine-Catalyzed Aminosulfonation of Hydrocarbons by Imidoiodinanes. A Synthetic and Mechanistic InvestigationDiogomussumNo ratings yet
- 1,3-Dioxanes, 1,3-DioxolanesDocument3 pages1,3-Dioxanes, 1,3-DioxolanesLaila Ambar SariNo ratings yet
- Synthesis and Regiochemistry of (60) Fullerenyl 2-Methylmalonate Bisadducts and Their Facile Electron-Accepting PropertiesDocument10 pagesSynthesis and Regiochemistry of (60) Fullerenyl 2-Methylmalonate Bisadducts and Their Facile Electron-Accepting PropertiesDiogo DiasNo ratings yet
- CO 301 Heterocyclic ChemistryDocument31 pagesCO 301 Heterocyclic ChemistryMohini BajajNo ratings yet
- Reaction MechanismDocument41 pagesReaction MechanismVarsha Dange88% (8)
- Multicomponent Reactions - Ambhaikar (July 2004)Document6 pagesMulticomponent Reactions - Ambhaikar (July 2004)muopioidreceptorNo ratings yet
- Regioselective N-9 Arylation of Purines Employing Arylboronic Acids in The Presence of Cu (II)Document4 pagesRegioselective N-9 Arylation of Purines Employing Arylboronic Acids in The Presence of Cu (II)Sreenath TrivikramNo ratings yet
- Musashi 2000Document11 pagesMusashi 2000DanCosminNo ratings yet
- JOC (1986) 51-4291 Ether DeprotDocument4 pagesJOC (1986) 51-4291 Ether DeprotludoNo ratings yet
- Synergistic Catalysis by Lewis Acid and Base Sites On Zro For Meerwein Ponndorf Verley ReductionDocument7 pagesSynergistic Catalysis by Lewis Acid and Base Sites On Zro For Meerwein Ponndorf Verley ReductionRiza SaidNo ratings yet
- StyreneDocument21 pagesStyrenewiam wiamNo ratings yet
- Zero-Valent Metals Accelerate The Neopentylglycolborylation of Aryl Halides Catalyzed by Nicl - Based Mixed-Ligand SystemsDocument7 pagesZero-Valent Metals Accelerate The Neopentylglycolborylation of Aryl Halides Catalyzed by Nicl - Based Mixed-Ligand SystemsDiogomussumNo ratings yet
- Palladium Catalysed Coupling ChemistryDocument16 pagesPalladium Catalysed Coupling ChemistryRah%No ratings yet
- Efficient Synthesis of 1,5-BenzodiazepinesDocument3 pagesEfficient Synthesis of 1,5-BenzodiazepinesdoubleffectNo ratings yet
- The Suzuki Cross-Coupling Final Version This Is ItDocument34 pagesThe Suzuki Cross-Coupling Final Version This Is ItBritany DyerNo ratings yet
- Coordination Chemistry—XIV: Plenary Lectures Presented at the XIVth International Conference on Coordination Chemistry Held at Toronto, Canada, 22—28 June 1972From EverandCoordination Chemistry—XIV: Plenary Lectures Presented at the XIVth International Conference on Coordination Chemistry Held at Toronto, Canada, 22—28 June 1972A. B. P. LeverNo ratings yet
- Unexpected Cleavage of 2-Azido-2 - (Hydroxymethyl) Oxetanes: Conformation Determines Reaction Pathway?Document8 pagesUnexpected Cleavage of 2-Azido-2 - (Hydroxymethyl) Oxetanes: Conformation Determines Reaction Pathway?DiogomussumNo ratings yet
- Of of A Z++, N3+, A13+-, To C-: 4 - (4-Methoxyphenyl) Butan-2-OneDocument4 pagesOf of A Z++, N3+, A13+-, To C-: 4 - (4-Methoxyphenyl) Butan-2-Onedyah erlienNo ratings yet
- Synlett: P. R. Krishna R. Sachwani P. S. ReddyDocument10 pagesSynlett: P. R. Krishna R. Sachwani P. S. Reddylydiem09No ratings yet
- Bencino 2Document44 pagesBencino 2José Emilio Román de AndaNo ratings yet
- Real InDictionary of Chemistry 4 BookDocument33 pagesReal InDictionary of Chemistry 4 Booktire farrokhzadNo ratings yet
- Creation of A Monomeric Ru Species On The Surface of Hydroxyapatite As An Efficient Heterogeneous Catalyst For Aerobic Alcohol OxidationDocument2 pagesCreation of A Monomeric Ru Species On The Surface of Hydroxyapatite As An Efficient Heterogeneous Catalyst For Aerobic Alcohol OxidationSveti JeronimNo ratings yet
- XXIIIrd International Congress of Pure and Applied Chemistry: Special Lectures Presented at Boston, USA, 26-30 July 1971From EverandXXIIIrd International Congress of Pure and Applied Chemistry: Special Lectures Presented at Boston, USA, 26-30 July 1971No ratings yet
- Chimie OrganicaDocument16 pagesChimie OrganicaDana CapbunNo ratings yet
- Zinc-Mediated Asymmetric Additions of Dialkylphosphine Oxides to r,β-Unsaturated Ketones and N-SulfinyliminesDocument8 pagesZinc-Mediated Asymmetric Additions of Dialkylphosphine Oxides to r,β-Unsaturated Ketones and N-SulfinyliminesDiogo DiasNo ratings yet
- Jo 01309 A 019Document7 pagesJo 01309 A 019Nagesh Babu KommojuNo ratings yet
- Palladium Catalyzed Cross CouplingDocument5 pagesPalladium Catalyzed Cross Couplingshiro_heNo ratings yet
- Enola TesDocument21 pagesEnola TesReksy WibowoNo ratings yet
- Titanium Dioxide Mediated Photocatalysed Degradation of Phenoxyacetic Acid and 2,4,5-Trichlorophenoxyacetic Acid, in Aqueous SuspensionsDocument7 pagesTitanium Dioxide Mediated Photocatalysed Degradation of Phenoxyacetic Acid and 2,4,5-Trichlorophenoxyacetic Acid, in Aqueous SuspensionsjionNo ratings yet
- 2005-Sonochemistry and Its DosimetryDocument6 pages2005-Sonochemistry and Its DosimetryOualid HamdaouiعععNo ratings yet
- 2005 OrgLett Highly Chemoselective Addition of Amines To Epoxides in WaterDocument3 pages2005 OrgLett Highly Chemoselective Addition of Amines To Epoxides in Waterjames mellaleievNo ratings yet
- 3 PHDocument4 pages3 PHamal aliNo ratings yet
- REdox Titration Intro and Non-Aqueous TitrationsDocument17 pagesREdox Titration Intro and Non-Aqueous TitrationsoyamaNo ratings yet
- Chem 115 Myers: Shi Asymmetric Epoxidation ReactionDocument5 pagesChem 115 Myers: Shi Asymmetric Epoxidation ReactionashisdasNo ratings yet
- s0040 40392961010 9Document4 pagess0040 40392961010 9Tudor UngureanNo ratings yet
- Chapter04 OxidationdDocument46 pagesChapter04 OxidationdWilliam H. BasingerNo ratings yet
- Transition Metal-Catalyzed Pyridine Synthesis: Transition Metal-Catalyzed Heterocycle Synthesis SeriesFrom EverandTransition Metal-Catalyzed Pyridine Synthesis: Transition Metal-Catalyzed Heterocycle Synthesis SeriesNo ratings yet
- 25th International Congress of Pure and Applied Chemistry: Plenary Lectures Presented at the 25th International Congress of Pure and Applied Chemistry, Jerusalem, Israel 6–11 July 1975From Everand25th International Congress of Pure and Applied Chemistry: Plenary Lectures Presented at the 25th International Congress of Pure and Applied Chemistry, Jerusalem, Israel 6–11 July 1975Rating: 5 out of 5 stars5/5 (1)
- Serial Review: Oxidative DNA Damage and Repair: Guest Editor: Miral DizdarogluDocument14 pagesSerial Review: Oxidative DNA Damage and Repair: Guest Editor: Miral DizdarogluRajathi YadavNo ratings yet
- AcSIR Syllabus1Document5 pagesAcSIR Syllabus1Rajathi YadavNo ratings yet
- Raman SpectrosDocument3 pagesRaman SpectrosRajathi YadavNo ratings yet
- Electrochemical Methods For Determination of Thymine in Medical Pipefish Samples Based On HPMFP/Ppy/GCE-modified ElectrodeDocument11 pagesElectrochemical Methods For Determination of Thymine in Medical Pipefish Samples Based On HPMFP/Ppy/GCE-modified ElectrodeRajathi YadavNo ratings yet
- PHD Student DetailsDocument1 pagePHD Student DetailsRajathi YadavNo ratings yet
- CHE-LAB-2-018 Advanced Materials Characterization Techniques: 2-0-0-2Document1 pageCHE-LAB-2-018 Advanced Materials Characterization Techniques: 2-0-0-2Rajathi YadavNo ratings yet
- Sensors and Biosensors For Endocrine Disrupting Chemicals: State-Of-The-Art and Future TrendsDocument36 pagesSensors and Biosensors For Endocrine Disrupting Chemicals: State-Of-The-Art and Future TrendsRajathi YadavNo ratings yet
- Carbohydrate Biosensors: Raz Jelinek and Sofiya KolushevaDocument30 pagesCarbohydrate Biosensors: Raz Jelinek and Sofiya KolushevaRajathi YadavNo ratings yet
- Cyclic Voltammetric Preparation of Palladium Nanoparticles For Ethanol Oxidation ReactionDocument6 pagesCyclic Voltammetric Preparation of Palladium Nanoparticles For Ethanol Oxidation ReactionRajathi YadavNo ratings yet
- Appl Electro ChemDocument29 pagesAppl Electro ChemRajathi YadavNo ratings yet
- Java PDFDocument235 pagesJava PDFRajathi YadavNo ratings yet
- Kang JihoDocument198 pagesKang JihoRajathi YadavNo ratings yet
- Teldir 29122011 Feb 232012Document39 pagesTeldir 29122011 Feb 232012Rajathi YadavNo ratings yet
- W3 02 Naming Chemical Formulas of CompoundsDocument20 pagesW3 02 Naming Chemical Formulas of CompoundsResmiel IrishNo ratings yet
- Pointers To Review 3PDocument9 pagesPointers To Review 3Phorace hernandezNo ratings yet
- Simple Harmonic MotionDocument29 pagesSimple Harmonic MotionthinkiitNo ratings yet
- FPSO Flow Meter Fits Just RightDocument1 pageFPSO Flow Meter Fits Just RightJuan JaramilloNo ratings yet
- 34 Samss 611Document10 pages34 Samss 611rizwan.zamanNo ratings yet
- Terahertz Metamaterials Perfect Absorbers For Sensing and ImagingDocument6 pagesTerahertz Metamaterials Perfect Absorbers For Sensing and ImaginghesoyamyecgaaaNo ratings yet
- Sound Grade 7Document1 pageSound Grade 7Bik BokNo ratings yet
- Secondary Steelmaking PaperDocument10 pagesSecondary Steelmaking Paperdebjit123No ratings yet
- Circular MotionDocument12 pagesCircular MotionSarthak ShingareNo ratings yet
- Lecture4 Characteristics of Covalent BondDocument33 pagesLecture4 Characteristics of Covalent BondMikhailah SigangNo ratings yet
- Chapter 9 Review AnswersDocument4 pagesChapter 9 Review AnswersHarrison LeeNo ratings yet
- Chemical Reactions - How Far and How FastDocument18 pagesChemical Reactions - How Far and How FastDoris GladiaNo ratings yet
- Genbio (Peta)Document18 pagesGenbio (Peta)Pauleen IldefonsoNo ratings yet
- Celestial Mechanics - Wikipedia PDFDocument5 pagesCelestial Mechanics - Wikipedia PDFgannwongNo ratings yet
- Practical Guide To Chlorate/perchlorate Electrolysis: WarningDocument14 pagesPractical Guide To Chlorate/perchlorate Electrolysis: WarningPink PantherNo ratings yet
- 3M 4279 Datasheet PDFDocument2 pages3M 4279 Datasheet PDFRehan SadiqNo ratings yet
- Emerson Chloride CP 70R Rectifier Charger 16 To 2500 An 3ph Inuput BrochueDocument2 pagesEmerson Chloride CP 70R Rectifier Charger 16 To 2500 An 3ph Inuput Brochuetimentitaek.mhdNo ratings yet
- Table of Fundamental Constants in Theoretical PhysicsDocument1 pageTable of Fundamental Constants in Theoretical PhysicsTunarisNo ratings yet
- Chemistry ProjectDocument13 pagesChemistry ProjectM100% (1)
- TEAM 1 - EV2 - LNatViDocument10 pagesTEAM 1 - EV2 - LNatViTamara PerezNo ratings yet
- Sp2 HybridizationDocument3 pagesSp2 HybridizationManP13No ratings yet
- Safety Data Sheet: Product Name: Mobil Aviation Grease SHC 100Document10 pagesSafety Data Sheet: Product Name: Mobil Aviation Grease SHC 100anibal_rios_rivasNo ratings yet
- AcidoBaseDrKellum PDFDocument84 pagesAcidoBaseDrKellum PDFalexander197100% (1)
- Food Preservation 3Document14 pagesFood Preservation 3Fhar AwayNo ratings yet
- Astm B571 97r08e1Document4 pagesAstm B571 97r08e1mspecsNo ratings yet
- Your Solution: For Nanoparticle Sizing and Zeta Potential MeasurementsDocument8 pagesYour Solution: For Nanoparticle Sizing and Zeta Potential MeasurementsRomain ThomasNo ratings yet
- ICH Q3A R2 - IMPURITIES IN NEW DRUG SUBSTANCES - Guia ICH para Determinação de Impurezas de Novas DrogasDocument15 pagesICH Q3A R2 - IMPURITIES IN NEW DRUG SUBSTANCES - Guia ICH para Determinação de Impurezas de Novas Drogasgrubensam100% (3)
- Solus Component CatalogDocument216 pagesSolus Component Catalognama saya kikoNo ratings yet
- 1.marie CurieDocument4 pages1.marie CurieEdgar Augusto Pedraza GomezNo ratings yet
- Atterberg Limit PDFDocument5 pagesAtterberg Limit PDFsanduni100% (1)