Professional Documents
Culture Documents
Boiling Point
Boiling Point
Uploaded by
Shanmuga MuthamilCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Boiling Point
Boiling Point
Uploaded by
Shanmuga MuthamilCopyright:
Available Formats
Boiling Point & Melting Point
X) Water are the factors influencing boiling point of Water? i) air pressure ii) Impurities
iv)the water is boiled at high pressure area Answer i) NO effect ii) NO effect iii) Impurities increase boiling point iv) Pressure increase, boiling point increase. 24) What are the factors influence evaporation? i) Surrounding temperature Evaporation rate ii) Humidty percentage Rate of evaporation iii) Surface area Rate of evaporation iv) Air speed Rate of evaporation v) Pressure Rate of evaporation 25)03088 Which one is true about absolute temperature? i) Same as 273 temperature ii) Freezing point of water iii) Temperature when mass of molecule turn to zero iv) Temperature when kinetic energy molecule is zero 28)03090 Water
add with salt B.P >100 ADD SALT,TEMP
pure B.P = 100
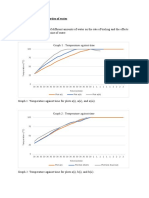
X2)03085 Boiling point of water can be increase by i) Increasing the rate of heating ii) Dilute substance that can dilute in water iii) Reduce humidity of air around water iv) Increasing the air pressure around the water. PROVE i)
Rate of heating Providing energy Reaching boiling point early. *But boiling point remain unchange Impurities add Humidity does not influence boiling point P boiling point
ii) iii)
iv)
23)03493 A student is heating up water and found that the water boilb at 140. Boiling point is more than 100 maybe because i)the water is boiled too long ii) volume of water boiled is to high iii)there is a bit of salt dilute in the water
You might also like
- Understanding Process Equipment for Operators and EngineersFrom EverandUnderstanding Process Equipment for Operators and EngineersRating: 4.5 out of 5 stars4.5/5 (3)
- MCQs (Elevation of Boiling Point)Document2 pagesMCQs (Elevation of Boiling Point)gamerwizcastNo ratings yet
- Week 3 - WaterDocument21 pagesWeek 3 - WaterChai LengNo ratings yet
- Boiling Heat TransferDocument12 pagesBoiling Heat TransferHarjith VaibavNo ratings yet
- IX-Deeksha (Liquid State)Document5 pagesIX-Deeksha (Liquid State)N. HarshaNo ratings yet
- HMT Boiling PPT (Autosaved)Document74 pagesHMT Boiling PPT (Autosaved)AVI NASHNo ratings yet
- EvaporationDocument41 pagesEvaporationGoodluck NyagybNo ratings yet
- Evaporation: Difference B/W Evaporation & BoilingDocument35 pagesEvaporation: Difference B/W Evaporation & BoilingSalim ChohanNo ratings yet
- Water Dew Point: From Citizendium, The Citizens' CompendiumDocument4 pagesWater Dew Point: From Citizendium, The Citizens' CompendiumJaime Ocampo SalgadoNo ratings yet
- Solutions and Solubility 2021Document3 pagesSolutions and Solubility 2021Mauro De LollisNo ratings yet
- Lecture 4Document12 pagesLecture 4bbfe89f31eNo ratings yet
- BoilingDocument65 pagesBoilingmuthu100% (1)
- EvaporationDocument4 pagesEvaporationCristian PavonNo ratings yet
- MCQ 9 1Document8 pagesMCQ 9 1Elixir ChemistryNo ratings yet
- Air Pollution and Global Warming (Lecture-3)Document23 pagesAir Pollution and Global Warming (Lecture-3)Sayed Mahbubul Hasan 1430589630No ratings yet
- Lecture 4 - Air Pollution and Global WarmingDocument23 pagesLecture 4 - Air Pollution and Global WarmingSafa Rabib RahmanNo ratings yet
- Air Pollution and Global Warming (Fall 2020)Document23 pagesAir Pollution and Global Warming (Fall 2020)Mohammad ArifuddozaNo ratings yet
- Physical Properties of WaterDocument4 pagesPhysical Properties of WaterLuk Ee RenNo ratings yet
- Changes of MatterDocument5 pagesChanges of MatterYaseenNo ratings yet
- 100 Questions On EvaporationDocument43 pages100 Questions On EvaporationFran Lee83% (6)
- Lab: Molar Heat of Vaporization and Fusion of WaterDocument1 pageLab: Molar Heat of Vaporization and Fusion of Waterkyle_tosh3382No ratings yet
- BBC Teachers Ks2 Science Worksheet Changing StateDocument6 pagesBBC Teachers Ks2 Science Worksheet Changing StateTotok SurotoNo ratings yet
- Equilibrium MCQ W AnsDocument6 pagesEquilibrium MCQ W AnsHovan Tall Nut Tan100% (1)
- Heat and Mass TransferDocument38 pagesHeat and Mass Transfersalahuddin khanNo ratings yet
- Solution LN Q& AnsDocument5 pagesSolution LN Q& AnsUva RaniNo ratings yet
- Physics em PDFDocument79 pagesPhysics em PDFD SiddaiahNo ratings yet
- 03a EvaporationDocument39 pages03a EvaporationAgustino WaitihacheNo ratings yet
- HHW Assignment IxDocument2 pagesHHW Assignment IxNaman YTNo ratings yet
- MCQs Physics Chap 6-9Document19 pagesMCQs Physics Chap 6-9Azka HaqNo ratings yet
- Boiling and CondensationDocument16 pagesBoiling and CondensationJunnaid NissarNo ratings yet
- SolutionDocument3 pagesSolutionUva RaniNo ratings yet
- Air Quality and Global WarmingDocument14 pagesAir Quality and Global WarmingAbdullah Al Muhaimin TohaNo ratings yet
- Mass Transfer HipalDocument24 pagesMass Transfer HipalsepticmoneyNo ratings yet
- Chapter 2 - SolutionsDocument10 pagesChapter 2 - SolutionsShubh MishraNo ratings yet
- Group 3 - Boiling Point ElevationDocument11 pagesGroup 3 - Boiling Point ElevationAnnissa PacaldoNo ratings yet
- Abstraction of PrecipitationDocument36 pagesAbstraction of PrecipitationRiot AyaseNo ratings yet
- Lec 2. Air Quality and Global Warming (28.6.21)Document16 pagesLec 2. Air Quality and Global Warming (28.6.21)Ehsan Munirus Saleheen 1812834030No ratings yet
- VaporpressureDocument14 pagesVaporpressureShaira TanNo ratings yet
- Abstraction From PrecipitationDocument28 pagesAbstraction From PrecipitationChristian LozadaNo ratings yet
- Van't Hoff FactorDocument17 pagesVan't Hoff FactorAnonymous OlpErbB7G100% (2)
- Matter in Our SurroundingDocument5 pagesMatter in Our SurroundingQSQFNo ratings yet
- Process Heat Transfer Lab: Dr. Farhan Javed (Assistant Professor)Document23 pagesProcess Heat Transfer Lab: Dr. Farhan Javed (Assistant Professor)nnbNo ratings yet
- Matter in Our Surroundings ClassDocument16 pagesMatter in Our Surroundings Classanuragmittal616No ratings yet
- 9 Boiling and Condensation 2024Document24 pages9 Boiling and Condensation 2024RAJAT RATHORENo ratings yet
- V Unit HTDocument7 pagesV Unit HTG Rakesh 18-343No ratings yet
- Class 9th Chemistry AssignmentDocument2 pagesClass 9th Chemistry AssignmentkittyroxxxNo ratings yet
- CHAPTER 5: EvaporationDocument10 pagesCHAPTER 5: EvaporationPRATIK AGAJNo ratings yet
- MCQ-9-1 MMDocument9 pagesMCQ-9-1 MMElixir ChemistryNo ratings yet
- Humidification: Review QuestionsDocument5 pagesHumidification: Review QuestionsJohn P. BandoquilloNo ratings yet
- Experiment 2Document7 pagesExperiment 2EDWIN SIMBARASHE MASUNUNGURENo ratings yet
- 12 Chemistry Exemplar Chapter 2 Answer PDFDocument4 pages12 Chemistry Exemplar Chapter 2 Answer PDFSorubh guptaNo ratings yet
- Grup Two PresentiestionDocument23 pagesGrup Two PresentiestionFira tubeNo ratings yet
- EASA AERODYNAMIC Module TESTDocument7 pagesEASA AERODYNAMIC Module TESTxristophorosNo ratings yet
- HT Unit-4Document12 pagesHT Unit-4MounikaMahalaxmi VelugubantlaNo ratings yet
- PIPE Elements 4 NIDocument88 pagesPIPE Elements 4 NIJd MagtibayNo ratings yet
- Matter in Our SurroundingDocument5 pagesMatter in Our SurroundingHimanshu TonkNo ratings yet
- Lecture 3 PDFDocument14 pagesLecture 3 PDFYousiff AliNo ratings yet
- Turbulence Phenomena: An Introduction to the Eddy Transfer of Momentum, Mass, and Heat, Particularly at InterfacesFrom EverandTurbulence Phenomena: An Introduction to the Eddy Transfer of Momentum, Mass, and Heat, Particularly at InterfacesNo ratings yet
- Case Studies in Fluid Mechanics with Sensitivities to Governing VariablesFrom EverandCase Studies in Fluid Mechanics with Sensitivities to Governing VariablesNo ratings yet
- CaltechDocument2 pagesCaltechShanmuga MuthamilNo ratings yet
- NuclearDocument1 pageNuclearShanmuga MuthamilNo ratings yet
- BookDocument1 pageBookShanmuga MuthamilNo ratings yet
- Must Go: Chichen ItzaDocument4 pagesMust Go: Chichen ItzaShanmuga MuthamilNo ratings yet
- Chemistry Form 4 Chapter 4Document6 pagesChemistry Form 4 Chapter 4Suriati Bt A Rashid100% (1)
- Chapter 3 Movement of Substances Across The Plasma MembraneDocument3 pagesChapter 3 Movement of Substances Across The Plasma MembraneShanmuga MuthamilNo ratings yet
- FOLIO: Biology Form 4 Chapter 2: Cell Structure and OrganisationDocument51 pagesFOLIO: Biology Form 4 Chapter 2: Cell Structure and OrganisationSashimi 刺身 Chebby100% (1)
- Places For SingaporeDocument21 pagesPlaces For SingaporeShanmuga MuthamilNo ratings yet