Professional Documents
Culture Documents
IGCSE Complete Physics Chapter 1
IGCSE Complete Physics Chapter 1
Uploaded by
shammah2rashadOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
IGCSE Complete Physics Chapter 1
IGCSE Complete Physics Chapter 1
Uploaded by
shammah2rashadCopyright:
Available Formats
10 m
number unit (m is the symbol for metre)
MEASUREMENTS AND UNITS
10
Numbers and units
1.01
When you make a measurement, you mi ght get a result li ke the one above: a
di stance of 10m. T he complete measurement i s called a physical quantity.
I t i s made up of two parts: a number and a uni t.
10m really means 10 m ( ten ti mes metre) , j ust as i n algebra, 10xmeans
10 x( ten ti mes x) . You can treat the m j ust li ke a symbol i n an algebrai c
equati on. T hi s i s i mportant when combi ni ng uni ts.
Combining units
I n the di agram above, the gi rl cycles 10 metres i n 2s. So she travels 5 metres
every second. H er speedi s 5 metres per second. T o work out the speed, you
di vi de the di stance travelled by the ti me taken, li ke thi s:
speed ( s i s the symbol for second)
A s m and s can be treated as algebrai c symbols:
speed
.
5
T o save space, 5 i s usually wri tten as 5m/s.
So m/s i s the uni t of speed.
Rights and wrongs
T hi s equati on i s correct: speed 5m/s
T hi s equati on i s i ncorrect: speed 5m/s
I t i s i ncorrect because the m and s have been left out. 10 di vi ded by 2 equals 5,
and not 5m/s.
Stri ctly speaki ng, uni ts should be i ncluded at all stages of a calculati on, not j ust
at the end. H owever, i n thi s book, the i ncorrect type of equati on wi ll someti mes
be used so that you can follow the ari thmeti c wi thout uni ts whi ch make the
calculati on look more compli cated.
m
s
10m
2s
10
2
m
s
m
s
10m
2s
10
2
Advanced units
5 m/s is a space-saving way of
writing 5 .
But 5 equals 5 m .
Also, can be written as s
-1
.
So the speed can be written as
5 ms
-1
.
This method of showing units is
more common in advanced work.
m
s
m
s
1
s
1
s
Q
MEASUREMENTS AND UNITS
11
1 H ow many gramsare there i n 1 ki logram?
2 H ow many mi lli metresare there i n 1 metre?
3 H ow many mi crosecondsare there i n 1 second?
4 T hi sequati on i sused to work out the area of a
rectangle: area length wi dth.
I f a rectangle measures3m by 2m, calculate i tsarea,
and i nclude the uni tsi n your calculati on.
5 Wri te down the followi ng i n km:
2000 m 200 m 2 10
4
m
6 Wri te down the followi ng i n s:
5000ms 5 10
7
s
7 U si ng sci enti c notati on, wri te down the followi ng to
two si gni cant gures:
1500m 1500000 m 0.15m 0.015m
Scientic notation
A n atlas says that the populati on of I celand i s thi s:
270000
T here are two problems wi th gi vi ng the number i n thi s form. Wri ti ng lots of
zeros i sn t very conveni ent. A lso, you don t know whi ch zeros are accurate. M ost
are only there to show you that i t i s a si x- gure number. T hese problems are
avoi ded i f the number i s wri tten usi ng powers of ten:
2.7 10
5
( 10
5
10 10 10 10 10 100000)
2.7 10
5
tells you that the gures 2 and 7 are i mportant. T he number i s bei ng
gi ven to two signicant gures. I f the populati on were known more accurately, to
three si gni cant gures, i t mi ght be wri tten li ke thi s:
2.70 10
5
N umbers wri tten usi ng powers of ten are i n scientic notationor standard
form. T he examples on the ri ght are to one si gni cant gure.
Bigger and smaller
You can make a uni t bi gger or smaller by putti ng an extra symbol, called a
pre x, i n front. ( Below, W stands for watt, a uni t of power.)
prex meaning example
G (giga) 1 000 000 000 (10
9
) GW (gigawatt)
M (mega) 1 000 000 (10
6
) MW (megawatt)
k (kilo) 1000 (10
3
) km (kilometre)
d (deci) (10
-1
) dm (decimetre)
c (centi) (10
-2
) cm (centimetre)
m (milli) (10
-3
) mm (millimetre)
(micro) (10
-6
) W (microwatt)
n (nano) (10
-9
) nm (nanometre)
Powers of 10
1000 10 x 10 x 10 10
3
100 10 x 10 10
2
0.1 10
-1
0.01 10
-2
0.001 10
-3
decimal fraction scientic
notation
500 5 x 10
2
0.5 5 x 10
-1
0.05 5 x 10
-2
0.005 5 x 10
-3
milli means thousandth,
not millionth
1
10
1
100
1
1000
1
10
3
1
10
2
5
10
5
100
5
1000
1
10
1
100
1
1000
1
1 000 000
1
1 000 000 000
Related topics: SI units 1.02; speed 2.01
Note: the SI base unit of mass is the kilogram, not the gram
mass
1 tonne (t)
1 kilogram (kg)
1 gram (g)
1 milligram (mg)
comparison with
base unit
scientific
notation
approximate size
medium-sized car
bag of sugar
banknote
human hair
1000 kg
1 kg
1 g
10
3
kg
10
-3
kg
10
-6
kg
1
1 000
kg
1
1 000
g
1
1 000 000
kg
MEASUREMENTS AND UNITS
12
A system of units
1.02
T here are many di fferent uni ts i ncludi ng those above. But i n sci enti c work,
li fe i s much easi er i f everyone uses a common system of uni ts.
SI units
M ost sci enti sts use SI units( full name: L e Systme I nternati onal d U ni ts) .
T he basi c SI uni ts for measuri ng mass, ti me, and length are the ki logram, the
second, and the metre. From these base unitscome a whole range of uni ts for
measuri ng volume, speed, force, energy, and other quanti ti es.
O ther SI base uni ts i nclude the ampere ( for measuri ng electri c current) and the
kelvi n ( for measuri ng temperature) .
Mass
M ass i s a measure of the amount of substance i n an obj ect. I t has two effects:
A ll obj ects are attracted to the Earth. T he greater the mass of an obj ect, the
stronger i s the Earths gravi tati onal pull on i t.
A ll obj ects resi st attempts to make them go faster, slower, or i n a di fferent
di recti on. T he greater the mass, the greater i s the resi stance to change i n
moti on.
T he SI base uni t of mass i s the kilogram( symbol kg) . T he standard ki logram i s
a block of plati num alloy kept at the O f ce of Wei ghts and M easures i n Pari s.
O ther uni ts based on the ki logram are shown below:
The mass of an object can be found
using a balancelike this. The balance
really detects the gravitational pull
on the object on the pan, but the
scale is marked to show the mass.
Mass Length Time
lb
oz
g
kg
ton
cwt
yd
cm
m
mile
ft
km
mm
s
hour
day
month
year
ms
Q
1 000 m
1 m
1
100
1
1 000
1
1 000 000
1
1 000 000 000
cm 1 2 3 4
mm 10 20 30 40
distance
1 kilometre (km) 10
3
m
10
2
m
10
3
m
10
6
m
10
9
m
1 metre (m)
1 centimetre (cm)
bacteria
atoms
1 millimetre (mm)
1 micrometre (m)
1 nanometre (nm)
comparison with
base unit
scientific
notation
approximate size
10 football pitches
m
m
m
m
Related topics: numbers and units 1.01; mass 2.07
MEASUREMENTS AND UNITS
13
1 What i sthe SI uni t of length?
2 What i sthe SI uni t of mass?
3 What i sthe SI uni t of ti me?
4 What do the followi ng symbolsstand for?
g mg t m ms
5 Wri te down the value of
a) 1564mm i n m b) 1750g i n kg
c) 26t i n kg d) 62si n s
e) 3.65 10
4
g i n kg f) 6.16 10
-7
mm i n m
6 T he 500 pagesof a book have a massof 2.50 kg. What
i sthe massof each page a) i n kg b) i n mg?
7 km g m t nm kg m
ms s mg ns s g mm
A rrange the above uni tsi n three columnsasbelow. T he
uni tsi n each column should be i n order, wi th the largest
at the top.
Time
T he SI base uni t of ti me i s the second( symbol s) . H ere are some shorter uni ts
based on the second:
1 mi lli second ( ms) s 10
-3
s
1 mi crosecond ( s) s 10
-6
s
1 nanosecond ( ns) s 10
-9
s
T o keep ti me, clocks and watches need somethi ng that beats at a steady rate.
Some old clocks used the swi ngs of a pendulum. M odern di gi tal watches count
the vi brati ons made by a ti ny quartz crystal.
Length
T he SI base uni t of length i s the metre( symbol m) . A t one ti me, the standard
metre was the di stance between two marks on a metal bar kept at the O f ce of
Wei ghts and M easures i n Pari s. A more accurate standard i s now used, based on
the speed of li ght, as on the ri ght.
T here are larger and smaller uni ts of length based on the metre:
The second was originally
dened as of a day,
one day being the time it takes
the Earth to rotate once. But the
Earths rotation is not quite
constant. So, for accuracy, the
second is now dened in terms
of something that never
changes: the frequency of an
oscillation which can occur in
the nucleus of a caesium atom.
By denition, one metre is the
distance travelled by light in a
vacuum in of a
second.
1
60 x 60 x 24
1
1000
1
1000000
1
1000000000
1
299 792 458
largest
unit
MEASUREMENTS AND UNITS
14
Measuring length and time
Measuring length
1.03
0
mm
10 20 30 40 50 60 70 80 90 100 110 120 130 140
L engths from a few mi llmetres up to a metre can be measured usi ng a rule,
as shown above. When usi ng the rule, the scale should be placed ri ght next to the
obj ect bei ng measured. I f thi s i s not possi ble, caliperscan be used, as shown on
the left. T he cali pers are set so that thei r poi nts exactly match the ends of the
obj ect. T hen they are moved across to a rule to make the measurement.
L engths of several metres can be measured usi ng a tapewi th a scale on i t.
Wi th small obj ects, more accurate length measurements can be made usi ng the
methods shown below:
Micrometer ( below left) T hi s has a revolvi ng barrel wi th an extra scale on i t.
T he barrel i s connected to a screw thread and, i n the example shown, each turn
of the barrel closes ( or opens) the gap by one mi lli metre. Fi rst, the gap i s opened
wi de. T hen i t i s closed up unti l the obj ect bei ng measured just ts i n i t ( a
cli cki ng sound i s heard) . T he di agram shows you how to take the readi ng.
Vernier calipers( below ri ght) T hi s i s an extra sli di ng scale tted to some
length-measuri ng i nstruments. I ts di vi si ons are set sli ghtly closer together than
normal so that one of them coi nci des wi th a di vi si on on the xed scale. T he
di agram shows you how to take the readi ng. ( T he verni er shown i s part of a set
of cali pers used for maki ng external measurements. A second type of cali per has
j aws for maki ng i nternal measurements.)
5.5 0.32
5.82 mm Add:
7 0.4
7.4 mm Add:
gap being
measured
fixed
scale
scale on
revolving barrel
gap being
measured
fixed
scale
mm 0 10 20
0 5
Read the highest scale
division that can be seen:
Read the scale on the
barrel, putting a decimal
point in front:
Read the highest scale
division before :
See where divisions coincide.
Read this on sliding scale,
putting a decimal point in front:
30
2
5
35
4
0
0 5
mm
Reading a micrometer Reading a vernier
calipers
If the rule cannot be placed next
to the object being measured,
calipers can be used.
Check and record your zero-error
reading and amend your answer
accordingly.
Related topics: units of length and time 1.02; timing a falling object 2.04
MEASUREMENTS AND UNITS
15
Measuring time
T i me i ntervals of many seconds or mi nutes can be measured usi ng a stopclock
or a stopwatch. Some i nstruments have an analoguedi splay, wi th a needle
( hand ) movi ng round a ci rcular scale. O thers have a digital di splay, whi ch
shows a number. T here are buttons for starti ng the ti mi ng, stoppi ng i t, and
resetti ng the i nstrument to zero.
Wi th a hand-operated stopclock or stopwatch, maki ng accurate measurements of
short ti me i ntervals ( a few seconds or less) can be di f cult. T hi s i s because of the
ti me i t takes you to react when you have to press the button. Fortunately, i n
some experi ments, there i s an si mple way of overcomi ng the problem. H ere i s an
example:
rigid support
string
bob
(small
mass)
simple
pendulum
one complete
swing
A pendulum can be set up to
investigate the time taken for
a single swing.
T he pendulum above takes about two seconds to make one complete swi ng.
Provi ded the swi ngs are small, every swi ng takes the same ti me. T hi s ti me i s
called i ts period. You can nd i t accurately by measuri ng the ti me for
25 swi ngs, and then di vi di ng the result by 25. For example:
T i me for 25 swi ngs = 55 seconds
So: ti me for 1 swi ng = 55/25 seconds = 2.2 seconds
A nother method of i mprovi ng accuracy i s to use automati c ti mi ng, as shown i n
the example on the ri ght. H ere, the ti me taken for a small obj ect to fall a short
di stance i s bei ng measured. T he ti mer i s started automati cally when the ball cuts
one li ght beam and stopped when i t cuts another.
1 A student measuresthe ti me taken for 20 swi ngsof a
pendulum. H e ndsthat the ti me taken i s46 seconds.
a) What ti me doesthe pendulum take for one swi ng?
b) H ow could the student have found the ti me for one
swi ng more accurately?
2 A student wantsto nd the thi cknessof one page of thi s
book. Explai n how she mi ght do thi saccurately.
3 A mi crometer i sused to measure the di ameter of a length of
copper wi re. T he zero error and scale readi ng are asshown.
a) What i sthe zero error of the mi crometer?
b) What i sthe correct di ameter of the wi re?
Q
15
10
5
0
40
45
0
0
45
40
35
30
25
20
0 5
zero error reading for copper wire
mm
mm
Measuring the time t it takes for a
steel ball to fall a distance h
time t
timer
electromagnet
to release ball
light
sensor
to start
timer
light
sensor
to stop
timer
steel
ball
h
Zero error
Vernier calipers are said to have
a zero errorif the zero marking
on the main scale is not in line
with the zero marking on the
vernier scale when the jaws are
fully closed. For example, on the
vernier calipers below, the zero
error is +0.02 cm.
If the scale reading = 4.09 cm
Zero error = 0.02 cm
Then, the corrected reading
= (4.09 0.02) = 4.07 cm
0
0
5
10
1
main scale
vernier scale
MEASUREMENTS AND UNITS
16
Volume and density
Volume
T he quanti ty of space an obj ect takes up i s called i ts volume.
T he SI uni t of volume i s the cubic metre( m
3
) . H owever, thi s i s rather large for
everyday work, so other uni ts are often used for conveni ence, as shown i n the
di agrams below:
1.04
Density
I s lead heavi er than water? N ot necessari ly. I t depends on the volumes of lead
and water bei ng compared. H owever, lead i s more dense than water: i t has more
ki lograms packed i nto every cubi c metre.
T he densityof a materi al i s calculated li ke thi s:
densi ty
I n the case of water:
a mass of 1000kg of water has a volume of 1m
3
a mass of 2000kg of water has a volume of 2m
3
a mass of 3000kg of water has a volume of 3m
3
, and so on.
U si ng any of these sets of gures i n the above equati on, the densi ty of water
works out to be 1000 kg/m
3
.
I f masses are measured i n grams ( g) and volumes i n cubi c centi metres ( cm
3
) , i t
i s si mpler to calculate densi ti es i n g/cm
3
. C onverti ng to kg/m
3
i s easy:
1g/cm
3
1000kg/m
3
T he densi ty of water i s 1g/cm
3
. T hi s si mple value i s no acci dent. T he ki logram
( 1000 g) was ori gi nally supposed to be the mass of 1000cm
3
of water ( pure, and
at 4
C ) . H owever, a very sli ght error was made i n the early measurement, so thi s
i s no longer used as a de ni ti on of the ki logram.
mass
volume
The glowing gas in the tail of a
comet stretches for millions of
kilometres behind the comets core.
The density of the gas is less than a
kilogram per cubic kilometre.
Cubic metre (m
3
)
1 cubic metre (m
3
) 1000 litres (l)
1 cubic metre (m
3
) is the volume of a
cube measuring 1 m 1 m 1 m.
Litre (l or L)
1 litre (L) 1000 cubic centimetres (cm
3
)
1000 millilitres (ml)
Note: the symbol
l for litre can be
confused with a
1 (one).
1 litre is the same volume as 1 cubic
decimetre (dm
3
)
Cubic centimetre (cm
3
)
or millilitre (ml or mL)
1 cubic centimetre (cm
3
) is
the volume of a cube
measuring 1 cm 1 cm 1cm.
It is the same volume as
1 millilitre (ml)
1 m
1 m
1 m
Q
V x
Related topics: pressure in liquids 3.06
MEASUREMENTS AND UNITS
17
1 H ow many cm
3
are there i n 1m
3
?
2 H ow many cm
3
are there i n 1li tre?
3 H ow many ml are there i n 1m
3
?
4 A tankful of li qui d hasa volume of 0.2m
3
. What i sthe
volume i n a) li tres b) cm
3
c) ml?
5 A lumi ni um hasa densi ty of 2700kg/m
3
.
a) What i sthe densi ty i n g/cm
3
?
b) What i sthe massof 20cm
3
of alumi ni um?
c) What i sthe volume of 27g of alumi ni um?
U se the i nformati on i n the table of densi ti esat the top of
the page to answer the followi ng:
6 What materi al, of mass39g, hasa volume of 5cm
3
?
7 What i sthe massof ai r i n a room measuri ng
5m 2m 3m?
8 What i sthe volume of a storage tank whi ch wi ll hold
3200kg of petrol?
9 What massof lead hasthe same volume as1600kg of
petrol?
Density calculations
T he equati on li nki ng densi ty, mass, and volume can be wri tten i n symbols:
where densi ty, m mass, and V volume
T hi s equati on can be rearranged to gi ve: V and m V
T hese are useful i f the densi ty i s known, but the volume or mass i s to be
calculated. O n the ri ght i s a method of ndi ng all three equati ons.
Example U si ng densi ty data from the table above, calculate the mass of steel
havi ng the same volume as 5400kg of alumi ni um.
Fi rst, calculate the volume of 5400kg of alumi ni um. I n thi s case,
i s 2700kg/m
3
, mi s 5400kg, and V i s to be found. So:
V 2m
3
T hi s i s also the volume of the steel. T herefore, for the steel, i s 7800 kg/m
3
, V i s
2 m
3
, and mi s to be found. So:
m V 7800kg/m
3
2m
3
15600kg
So the mass of steel i s 15600kg.
5400kg
2700kg/m
3
m
m
V
Cover V in the triangle and you can
see what V is equal to. It works for m
and as well.
substance density density
kg/m
3
g/cm
3
air 1.3 0.0013
expanded polystyrene 14 0.014
wood (beech) 750 0.75
petrol 800 0.80
ice (0 C) 920 0.92
polythene 950 0.95
water (4 C) 1000 1.0
concrete 2400 2.4
glass (varies) 2500 2.5
substance density density
kg/m
3
g/cm
3
granite 2700 2.7
aluminium 2700 2.7
steel (stainless) 7800 7.8
copper 8900 8.9
lead 11 400 11.4
mercury 13 600 13.6
gold 19 300 19.3
platinum 21 500 21.5
osmium 22 600 22.6
The rare metal osmium is the
densest substance found on
Earth. If this book were made of
osmium, it would weigh as
much as a heavy suitcase.
The densities of solids and
liquids vary slightly with
temperature. Most substances
get a little bigger when heated.
The increase in volume reduces
the density.
The densities of gases can vary
enormously depending on how
compressed they are.
In the density equation, the
symbol is the Greek letter
rho.
measuring
cylinder
level on scale
gives volume
of liquid
1000 cm
3
increase
in level
gives
volume
of solid
1000 cm
3
1000 cm
3
MEASUREMENTS AND UNITS
18
Measuring volume and density
Measuring volume
Liquid A volume of about a li tre or so can be measured usi ng a measuri ng
cyli nder. When the li qui d i s poured i nto the cyli nder, the level on the scale gi ves
the volume.
M ost measuri ng cyli nders have scales marked i n mi lli li tres ( ml) , or cubi c
centi metres ( cm
3
) .
Regular solid I f an obj ect has a si mple shape, i ts volume can be calculated. For
example:
volume of a rectangular block length wi dth hei ght
volume of a cyli nder ! radi us
2
hei ght
Irregular solid I f the shape i s too awkward for the volume to be calculated, the
soli d can be lowered i nto a partly lled measuri ng cyli nder as shown on the left.
T he risei n level on the volume scale gi ves the volume of the soli d.
I f the soli d oats, i t can be wei ghed down wi th a lump of metal. T he total
volume i s found. T he volume of the metal i s measured i n a separate experi ment
and then subtracted from thi s total.
Using a displacement can I f the soli d i s too bi g for a measuri ng cyli nder, i ts
volume can be found usi ng a di splacement can, shown below left. Fi rst, the can
i s lled up to the level of the spout ( thi s i s done by over lli ng i t, and then wai ti ng
for the surplus water to run out) . T hen the soli d i s slowly lowered i nto the water.
T he soli d i s now taki ng up space once occupi ed by the water i n other words, i t
has di splaced i ts own volume of water. T he di splaced water i s collected i n a
beaker and empti ed i nto a measuri ng cyli nder.
T he di splacement method, so the story goes, was di scovered by acci dent, by
A rchi medes. You can nd out how on the opposi te page.
Measuring density
T he densi ty of a materi al can be found by calculati on, once the volume and mass
have been measured. T he mass of a small soli d or of a li qui d can be measured
usi ng a balance. H owever, i n the case of a li qui d, you must remember to allow
for the mass of i ts contai ner.
H ere are some readi ngs from an experi ment to nd the densi ty of a li qui d:
1.05
Measuring the volume of a small
solid
Measuring the volume of a liquid
T herefore: mass of li qui d 560 g 240 g 320 g ( C B)
T herefore densi ty of li qui d 0.8 g/cm
3
volume of liquid in measuring cylinder = 400 cm
3
( A )
mass of measuring cylinder = 240 g ( B)
mass of measuring cylinder with liquid in = 560 g ( C )
mass
volume
320 g
400 cm
3
Using a displacement can. Provided
the can is lled to the spout at the
start, the volume of water collected
in the beaker is equal to the volume
of the object lowered into the can.
Q
mass/ g
density: gold 19.3 g/cm
3
; silver 10.5 g/cm
3
volume/ cm
3
crown A
3750
357
3750
194
crown B
3750
315
crown C
liquid added
148
cm
3
290 g 90 g
100
cm
3
empty stone added
170 g
Related topics: volume and density 1.04
MEASUREMENTS AND UNITS
19
1 U se the i nformati on above to deci de whi ch crown i s
gold, whi ch i ssi lver, and whi ch i sa mi xture.
2 U se the i nformati on above to calculate:
a) the mass, volume, and densi ty of the li qui d,
b) the mass, volume, and densi ty of the stone.
Relative density*
T he relati ve densi ty of a substance tells you how the densi ty compares wi th that
of water. I t i s calculated li ke thi s:
relati ve densi ty
For example, lead has a densi ty of 11300kg/m
3
( 11.3g/cm
3
) and water has a
densi ty of 1000kg/m
3
( 1g/cm
3
) . So:
relati ve densi ty of lead 11.3
R elati ve densi ty has no uni ts. I t i s a number whose value i s the same as that of
the densi ty i n g/cm
3
. I t used to be known as speci c gravi ty .
11.3g/cm
3
1g/cm
3
11300kg/m
3
1000kg/m
3
densi ty of substance
densi ty of water
substance density relative
density
water 1.0 g/cm
3
1.0
aluminium 2.7 g/cm
3
2.7
copper 8.9 g/cm
3
8.9
lead 11.4 g/cm
3
11.4
mercury 13.6 g/cm
3
13.6
gold 19.3 g/cm
3
19.3
Archimedes and the crown
Archimedes, a Greek mathematician, lived in Syracuse
(now in Sicily) around 250 BC. He made important
discoveries about levers and liquids, but is probably best
remembered for his clever solution to a problem set him by
the King of Syracuse.
The King had given his goldsmith some gold to make
a crown. But when the crown was delivered, the King
was suspicious. Perhaps the goldsmith had stolen
some of the gold and mixed in cheaper silver
instead. The King asked Archimedes to test the crown.
Archimedes knew that the crown was the correct mass. He also knew that silver
was less dense than gold. So a crown with silver in it would have a greater volume
than it should have. But how could he measure the volume? Stepping into his bath
one day, so the story goes, Archimedes noticed the rise in water level. Here was the
answer! He was so excited that he lept from his bath and ran naked through the streets,
shouting Eureka! , which means I have found it! .
Later, Archimedes put the crown in a container of water and measured the rise in level.
Then he did the same with an equal mass of pure gold. The rise in level was different. So
the crown could not have been pure gold.
MEASUREMENTS AND UNITS
20
More about mass and density
Comparing masses
T he devi ce above i s called a beam balance. I t i s the si mplest, and probably the
oldest, way of ndi ng the mass of somethi ng. You put the obj ect i n one pan, then
add standard masses to the other pan unti l the beam balances i n a level posi ti on.
I f you have to add 1.2 kg of standard masses, as i n the di agram, then you know
that the obj ect also has a mass of 1.2 kg.
T he balance i s really compari ng wei ghts rather than masses. Wei ght i s the
downward pull of gravi ty. T he beam balances when the downward pull on one
pan i s equal to the downward pull on the other. H owever, masses can be
compared because of the way gravi ty acts on them. I f the obj ects i n the two pans
have the same wei ght, they must also have the same mass.
When usi ng a balance li ke the one above, you mi ght say that you were wei ghi ng
somethi ng. H owever, 1.2 kg i s the mass of the obj ect, not i ts wei ght. Wei ght i s a
force, measured i n force uni ts called newtons. For more on thi s, and the
di fference between mass and wei ght, see spreads 2.07 and 2.09.
A more modern type of balance i s shown on the left.
1.06
Q
1 O n the M oon, the force of gravi ty on an obj ect i sonly
about one si xth of i tsvalue on Earth. D eci de whether
each of the followi ng would gi ve an accurate
measurement of massi f used on the M oon.
a) A beam balance li ke the one i n the di agram at the top
of the page.
b) A balance li ke the one i n the photograph above.
2 A balloon li ke the one on the opposi te page contai ns
2000 m
3
of ai r. When the ai r i scold, i tsdensi ty i s
1.3 kg/m
3
. When heated, the ai r expandsso that some i s
pushed out of the hole at the bottom, and the densi ty falls
to 1.1 kg/m
3
. C alculate the followi ng.
a) T he massof ai r i n the balloon when cold.
b) T he massof ai r i n the balloon when hot.
c) T he massof ai r lost from the balloon duri ng heati ng.
beam
pan pan
unknown mass
standard masses
200 g
500 g
500 g
A simple beam balance
A more modern type of balance. It
detects the gravitational pull on the
object on the pan, but gives its
reading in units of mass.
Density essentials
density .
mass
volume
Related topics: mass 1.02; volume and density 1.04105; force 2.06; mass and weight 2.09; convection 5.07
MEASUREMENTS AND UNITS
21
Planet density
The density of a planet increases towards the centre. However, the average
density can be found by dividing the total mass by the total volume. The mass
of a planet affects its gravitational pull and, therefore, the orbit of any moon
circling it. The mass can be calculated from this. The volume can be calculated
once the diameter is known.
The average density gives clues about a planets structure:
Earth
Average density 5520 kg/m
3
This is about double the density of the
rocks near the surface, so the Earth
must have a high density core
probably mainly iron.
Jupiter
Average density 1330 kg/m
3
The low average density is one reason
why scientists think that Jupiter is a
sphere mostly of hydrogen and helium
gas, with a small, rocky core. not to scale
Float or sink?
You can tell whether a material will oat or sink by comparing its density with that of the
surrounding liquid (or gas). If it is less dense, it will oat; if it is more dense, it will sink. For
example, wood is less dense than water, so it oats; steel is more dense, so it sinks.
Density differences are not the causeof oating or sinking, just a useful guide for predicting
which will occur. Floating is made possible by an upward force produced whenever an
object is immersed in a liquid (or gas). To experience this force, try pushing an empty bottle
down into water.
Ice is less dense than water in its liquid form, so icebergs oat.
Hot air is less dense than cold air,
so a hot-air balloon will rise
upwards provided the fabric, gas
cylinders, basket, and passengers
do not increase the average density
by too much.
MEASUREMENTS AND UNITS
1 C opy and complete the table shown below:
[ 6]
2 Wri te down the number of
A mg i n 1 g
B g i n 1 kg
C mg i n 1 kg
D mm i n 4 km
E cm i n 5 km [ 5]
3 Wri te down the valuesof
a) 300 cm, i n m
b) 500 g, i n kg
c) 1500 m, i n km
d) 250 ms, i n s
e) 0.5 s, i n ms
f) 0.75 km, i n m
g) 2.5 kg, i n g
h) 0.8 m, i n mm [ 8]
4 T he volume of a rectangular block can be calculated
usi ng thi sequati on:
volume length wi dth hei ght
U si ng thi si nformati on, copy and complete the table
below. [ 4]
5 I n each of the followi ng pai rs, whi ch quanti ty i sthe larger?
a) 2km or 2500m?
b) 2m or 1500mm?
c) 2 tonnesor 3000kg?
d) 2 li tresor 300cm
3
? [ 4]
EXAMINATION QUESTIONS
22
measurement unit symbol
length
?
?
?
kilogram
?
?
?
s
length width height
volumeof
rectangular block
2 cm
5 cm
6 cm
?
3 cm
5 cm
?
10 cm
4 cm
?
5 cm
10 cm
?
100 cm
3
300 cm
3
50 cm
3
6 Whi ch of the followi ng statementsi s/are correct?
A O ne mi lli gram equalsone mi lli on grams.
B O ne thousand mi lli gramsequalsone gram.
C O ne mi lli on mi lli gramsequalsone gram.
D O ne mi lli on mi lli gramsequalsone ki logram. [ 2]
7 m g/cm
3
m
3
km cm
3
kg ms ml kg/m
3
s
Whi ch of the above are
a) uni tsof mass?
b) uni tsof length
c) uni tsof volume?
d) uni tsof ti me?
e) uni tsof densi ty? [ 11]
8 Whi ch block i smade of the densest materi al?
9 T he massof a measuri ng cyli nder and i tscontentsare
measured before and after putti ng a stone i n i t.
Whi ch of the followi ng could you calculate usi ng
measurementstaken from the apparatusabove?
A the densi ty of the li qui d only
B the densi ty of the stone only
C the densi ti esof the li qui d and the stone [ 2]
10 A plasti c bag lled wi th ai r hasa volume of 0.008m
3
.
When ai r i n the bag i ssqueezed i nto a ri gi d contai ner, the
massof the contai ner ( wi th ai r) i ncreasesfrom 0.02kg to
0.03kg. U se the formula
densi ty
to calculate the densi ty of the ai r i n the bag. [ 2]
11
mass
volume
block mass/g length/cm breadth/cm height/cm
A 480 5 4 4
B 360 10 4 3
C 800 10 5 2
D 600 5 4 3
measuring
cylinder
same volume
of water
balance
stone
12 T he table showsthe densi ty of vari oussubstances.
substance density/ g/cm
3
copper 8.9
i ron 7.9
kerosene 0.87
mercury 13.6
water 1.0
C onsi der the followi ng statements:
A 1cm
3
of mercury hasa greater massthan 1cm
3
of any
other substance i n thi stable true or false?
B 1cm
3
of water hasa smaller massthan 1cm
3
of any
other substance i n thi stable true or false?
C 1g of i ron hasa smaller volume than 1g of copper
true or false?
D 1g of mercury hasa greater massthan 1g of copper
true or false? [ 2]
13 A student deci desto measure the peri od of a pendulum
( the peri od i sthe ti me taken for one complete swi ng) .
U si ng a stopwatch, he ndsthat 8 complete swi ngstake
7.4 seconds. Wi th hi scalculator, he then usesthi sdata
to work out the ti me for one swi ng. T he number shown
on hi scalculator i s0.925.
a) I si t acceptable for the student to clai m that the
peri od of the pendulum i s0.925 seconds? Explai n your
answer. [ 2]
b) H ow could the student measure the peri od more
accurately? [ 2]
c) L ater, another student ndsthat 100 complete swi ngs
take 92.8 seconds. From these measurements, what i s
the peri od of the pendulum? [ 2]
EXAMINATION QUESTIONS
MEASUREMENTS AND UNITS
23
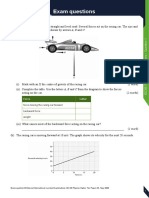
I n the di agram above, the tankscontai n two di fferent
li qui ds, X and Y.
a) What i sthe volume of each li qui d i n m
3
? [ 2]
b) I f you had 1 m
3
of the li qui d X , what would i ts
massbe? [ 2]
c) What i sthe densi ty of li qui d X ? [ 2]
d) What i sthe densi ty of li qui d Y ? [ 2]
0.4 m
0.5 m
0.5 m
0.2 m
0.5 m
0.5 m
liquid Y
mass 50 kg
liquid X
mass 80 kg
MEASUREMENTS AND UNITS
24
REVISION CHECKL IST
OUP this page may be photocopied solely by the purchasers institute
1 H ow to use uni ts. ( 1.01)
2 M aki ng bi gger or smaller uni ts usi ng
pre xes. ( 1.01)
3 Wri ti ng numbers i n sci enti c notati on. ( 1.01)
4 Si gni cant gures. ( 1.01)
5 SI uni ts, i ncludi ng the metre, ki logram, and
second. ( 1.02)
6 What cali pers are used for. ( 1.03)
7 H ow to read a mi crometer. ( 1.03)
8 H ow to read a verni er scale. ( 1.03)
9 H ow to measure short i ntervals of ti me. ( 1.03)
10 H ow to nd the peri od of a si mple
pendulum. ( 1.03)
11 T he meani ng of zero error. ( 1.03)
12 U ni ts for measuri ng volume. ( 1.04)
13 H ow densi ty i s de ned. ( 1.04)
14 U si ng the equati on li nki ng mass, volume,
and densi ty. ( 1.04)
15 M ethods of measuri ng the volume of
a li qui d
a regular soli d
an i rregular soli d. ( 1.05)
16 H ow to use a di splacement can. ( 1.05)
17 T he meani ng of relati ve densi ty. ( 1.05)
18 H ow to compare masses wi th a beam
balance. ( 1.06)
Photocopy the li st of topi cs below and ti ck the boxes of the ones that are
i ncluded i n your exami nati on syllabus. ( Your teacher should be able to tell you
whi ch they are.) U se your li st when you revi se. T he spread number i n brackets
tells you where to nd more i nformati on.
25
A
bungee jumper leaps more than 180 metres from the top of
the S ky Tower in Auckland, New Zealand. With nothing to
oppose his fall, he would hit the ground at a speed of 60
metres per second. However, his fall is slowed by the resistance of the air
rushing past him, and eventually stopped by the pull of the bungee rope.
S ide ropes are also being used to stop him crashing into the tower.
2
A
bungee jumper leaps more than 180 metres from the top of
the S ky Tower in Auckland, New Zealand. With nothing to
oppose his fall, he would hit the ground at a speed of 60
metres per second. However, his fall is slowed by the resistance of the air
rushing past him, and eventually stopped by the pull of the bungee rope.
S ide ropes are also being used to stop him crashing into the tower.
SPEED AND VELOCITY
ACCELERATION
FREEFALL
FORCEAND MASS
FRICTION AND BRAKING
GRAVITY AND WEIGHT
ACTION AND REACTION
VECTORSAND SCALARS
CIRCULARMOTION
SPEED AND VELOCITY
ACCELERATION
FREEFALL
FORCEAND MASS
FRICTION AND BRAKING
GRAVITY AND WEIGHT
ACTION AND REACTION
VECTORSAND SCALARS
CIRCULARMOTION
25
You might also like
- IGCSE Physics - DensityDocument12 pagesIGCSE Physics - DensityEdgardo Leysa90% (29)
- IGCSE Physics Worksheet-MeasurementDocument8 pagesIGCSE Physics Worksheet-Measurementgdsuta88% (42)
- Pressure Questions For IGCSE PhysicsDocument14 pagesPressure Questions For IGCSE PhysicsMohamed Jameel77% (31)
- IGCSE Physics - Length and TimeDocument14 pagesIGCSE Physics - Length and TimeEdgardo Leysa91% (33)
- Year11 IGCSE Physics Scheme of WorkDocument20 pagesYear11 IGCSE Physics Scheme of WorkTiras Ngugi100% (1)
- CIE IGCSE Physics (0625) Pressure NotesDocument22 pagesCIE IGCSE Physics (0625) Pressure Notes9322483% (53)
- Physics Paper 6 Revision NotesDocument5 pagesPhysics Paper 6 Revision NotesAmalia Korakaki88% (17)
- Mid-Term IGCSE PhysicsDocument4 pagesMid-Term IGCSE Physicsgdsuta89% (9)
- Grade 3 Maths Worksheets Word Problems On Grams and Kilograms 8Document2 pagesGrade 3 Maths Worksheets Word Problems On Grams and Kilograms 8Anonymous so6ZnlKyw100% (3)
- Teacher Guide: Cambridge IGCSE / Cambridge IGCSE (9-1) Physics 0625 / 0972Document28 pagesTeacher Guide: Cambridge IGCSE / Cambridge IGCSE (9-1) Physics 0625 / 0972Aditya Rao100% (1)
- IGCSE Physics FormulasDocument1 pageIGCSE Physics FormulasPawan Kolhe80% (30)
- IGCSE Physics Exam Revision NotesDocument34 pagesIGCSE Physics Exam Revision NotesCoolman Poon88% (17)
- Cambridge IGCSE Physics Practice Book AnswersDocument22 pagesCambridge IGCSE Physics Practice Book AnswersRonnie Quek50% (2)
- Cambridge IGCSE Physics Teacher PackDocument19 pagesCambridge IGCSE Physics Teacher Packbiofire75% (8)
- IGCSE Physics Revison Question BankDocument43 pagesIGCSE Physics Revison Question BankSyed Waqas Ahmed100% (4)
- Igcse Physics Short NotesDocument56 pagesIgcse Physics Short NotesakashNo ratings yet
- IGCSE TrigonometryDocument14 pagesIGCSE TrigonometryDienta Alias100% (10)
- Estimation of MagDocument6 pagesEstimation of MagDeepak ShrivastavNo ratings yet
- Physics Atp TipsDocument2 pagesPhysics Atp TipsFehmeed Alchemy78% (9)
- Igcse Physics RevisionDocument44 pagesIgcse Physics Revisionlozzzzz88% (17)
- 12 - LightDocument25 pages12 - LightEdgardo LeysaNo ratings yet
- IGCSE Thermal Physics WorksheetDocument1 pageIGCSE Thermal Physics Worksheetgdsuta67% (3)
- 13 - SoundDocument22 pages13 - SoundEdgardo Leysa100% (1)
- IGCSE Physics - Mass & WeightDocument17 pagesIGCSE Physics - Mass & WeightEdgardo Leysa100% (11)
- Mid-Term IGCSE PhysicsDocument9 pagesMid-Term IGCSE Physicsgdsuta80% (5)
- Cambridge iGCSE Physics Formula Sheet - Teaching Resources PDFDocument6 pagesCambridge iGCSE Physics Formula Sheet - Teaching Resources PDFMalak Mqq100% (2)
- Physics IgcseDocument36 pagesPhysics IgcseLilly Swift89% (9)
- IGCSE Physics MomentsDocument3 pagesIGCSE Physics MomentsKasunDilshanNo ratings yet
- IGCSE Physics Worksheet 3.3Document1 pageIGCSE Physics Worksheet 3.3Alex DatsyukNo ratings yet
- RadioactivityDocument16 pagesRadioactivityEdgardo Leysa100% (1)
- Electromagnetic Effects: 300 Turns 30 TurnsDocument23 pagesElectromagnetic Effects: 300 Turns 30 TurnsEdgardo Leysa100% (5)
- Form 4 - IGCSE Physics - ThermometersDocument13 pagesForm 4 - IGCSE Physics - ThermometersMr. Borges93% (57)
- Gce A and As Level Physics Topical Past Papers 1976 To 2003 PDFDocument388 pagesGce A and As Level Physics Topical Past Papers 1976 To 2003 PDFWaleed Bin Khalid100% (1)
- As Level Physics Practical Paper 3Document7 pagesAs Level Physics Practical Paper 3Asif AyazNo ratings yet
- 83 Revision Questions For IGCSE Questions Solutions PDFDocument5 pages83 Revision Questions For IGCSE Questions Solutions PDFChui Wai100% (4)
- IGCSE Physics Paper 1Document182 pagesIGCSE Physics Paper 1Hakim Abbas Ali Phalasiya100% (24)
- 22 - The Nuclear AtomDocument10 pages22 - The Nuclear AtomEdgardo LeysaNo ratings yet
- Handout For Physics IGCSEDocument28 pagesHandout For Physics IGCSEDewi Niken Ariyanti100% (1)
- IGCSE Physics NotesDocument6 pagesIGCSE Physics Noteslhngu0% (1)
- iGCSE Physics Formula Sheet: MotionDocument4 pagesiGCSE Physics Formula Sheet: MotionYusuf100% (3)
- IGCSE Physics NotesDocument16 pagesIGCSE Physics NotesMhdAboualy84% (32)
- Unit Physics WorksheetsDocument10 pagesUnit Physics WorksheetsVarshLok100% (2)
- Thermal Physics CIE IGCSE 0625 PPQDocument11 pagesThermal Physics CIE IGCSE 0625 PPQPeter IsigiNo ratings yet
- O level Physics Questions And Answer Practice Papers 1From EverandO level Physics Questions And Answer Practice Papers 1Rating: 3.5 out of 5 stars3.5/5 (4)
- O level Physics Questions And Answer Practice Papers 3From EverandO level Physics Questions And Answer Practice Papers 3Rating: 3 out of 5 stars3/5 (1)
- 1.5 Scales Problems and SolutionsDocument21 pages1.5 Scales Problems and SolutionsHimanshu Gupta75% (4)
- 1.5 Scales Problems and SolutionsDocument21 pages1.5 Scales Problems and SolutionsmaxNo ratings yet
- How UnitDocument2 pagesHow UnitHatdogNo ratings yet
- General PhysicsDocument43 pagesGeneral PhysicsDhanica B. FiguracionNo ratings yet
- Conversion... Units And... Derived Units-1Document53 pagesConversion... Units And... Derived Units-1svenkatk737No ratings yet
- Part1 Mechanics of Point Particles: 1.2 Working With Numbers 1.3 Si Unit SystemDocument20 pagesPart1 Mechanics of Point Particles: 1.2 Working With Numbers 1.3 Si Unit Systemsara fekriNo ratings yet
- Conversion... Units And... Derived Units-1 (2) (3) - 1Document49 pagesConversion... Units And... Derived Units-1 (2) (3) - 1svenkatk737No ratings yet
- 0620 w14 QP 11Document16 pages0620 w14 QP 11Haider Ali100% (1)
- 0620 w14 QP 61Document12 pages0620 w14 QP 61Haider AliNo ratings yet
- 0620 w14 QP 63Document12 pages0620 w14 QP 63Haider AliNo ratings yet
- 0610 w14 QP 32Document24 pages0610 w14 QP 32Haider AliNo ratings yet
- PHP 5 Classes and ObjectsDocument39 pagesPHP 5 Classes and ObjectsHubbak KhanNo ratings yet
- University of Cambridge International Examinations General Certificate of Education Advanced LevelDocument4 pagesUniversity of Cambridge International Examinations General Certificate of Education Advanced LevelHubbak KhanNo ratings yet
- IBCC Equilence Form For O/A Levels.Document5 pagesIBCC Equilence Form For O/A Levels.Hubbak Khan100% (1)
- 0620 w14 QP 62Document12 pages0620 w14 QP 62Haider AliNo ratings yet
- University of Cambridge International Examinations General Certificate of Education Advanced Subsidiary Level and Advanced LevelDocument4 pagesUniversity of Cambridge International Examinations General Certificate of Education Advanced Subsidiary Level and Advanced LevelHubbak KhanNo ratings yet
- University of Cambridge International Examinations General Certificate of Education Advanced LevelDocument4 pagesUniversity of Cambridge International Examinations General Certificate of Education Advanced LevelHubbak KhanNo ratings yet
- University of Cambridge International Examinations General Certificate of Education Advanced Subsidiary Level and Advanced LevelDocument4 pagesUniversity of Cambridge International Examinations General Certificate of Education Advanced Subsidiary Level and Advanced LevelHubbak KhanNo ratings yet
- University of Cambridge International Examinations General Certificate of Education Advanced LevelDocument4 pagesUniversity of Cambridge International Examinations General Certificate of Education Advanced LevelHubbak KhanNo ratings yet
- University of Cambridge International Examinations General Certificate of Education Advanced LevelDocument4 pagesUniversity of Cambridge International Examinations General Certificate of Education Advanced LevelHubbak KhanNo ratings yet
- University of Cambridge International Examinations General Certificate of Education Advanced Subsidiary Level and Advanced LevelDocument4 pagesUniversity of Cambridge International Examinations General Certificate of Education Advanced Subsidiary Level and Advanced LevelHubbak KhanNo ratings yet
- University of Cambridge International Examinations General Certificate of Education Advanced LevelDocument4 pagesUniversity of Cambridge International Examinations General Certificate of Education Advanced LevelHubbak KhanNo ratings yet
- 9709 s12 QP 21 PDFDocument4 pages9709 s12 QP 21 PDFHubbak KhanNo ratings yet
- University of Cambridge International Examinations General Certificate of Education Advanced Subsidiary Level and Advanced LevelDocument4 pagesUniversity of Cambridge International Examinations General Certificate of Education Advanced Subsidiary Level and Advanced LevelHubbak KhanNo ratings yet
- University of Cambridge International Examinations General Certificate of Education Advanced LevelDocument24 pagesUniversity of Cambridge International Examinations General Certificate of Education Advanced LevelHubbak KhanNo ratings yet
- University of Cambridge International Examinations General Certificate of Education Advanced LevelDocument4 pagesUniversity of Cambridge International Examinations General Certificate of Education Advanced LevelHubbak KhanNo ratings yet
- 9709 s12 QP 21 PDFDocument4 pages9709 s12 QP 21 PDFHubbak KhanNo ratings yet
- University of Cambridge International Examinations General Certificate of Education Advanced LevelDocument8 pagesUniversity of Cambridge International Examinations General Certificate of Education Advanced LevelHubbak KhanNo ratings yet
- University of Cambridge International Examinations General Certificate of Education Advanced LevelDocument8 pagesUniversity of Cambridge International Examinations General Certificate of Education Advanced LevelHubbak KhanNo ratings yet
- University of Cambridge International Examinations General Certificate of Education Advanced Subsidiary Level and Advanced LevelDocument16 pagesUniversity of Cambridge International Examinations General Certificate of Education Advanced Subsidiary Level and Advanced LevelHubbak KhanNo ratings yet
- University of Cambridge International Examinations General Certificate of Education Advanced Subsidiary Level and Advanced LevelDocument16 pagesUniversity of Cambridge International Examinations General Certificate of Education Advanced Subsidiary Level and Advanced LevelHubbak KhanNo ratings yet
- University of Cambridge International Examinations General Certificate of Education Advanced LevelDocument24 pagesUniversity of Cambridge International Examinations General Certificate of Education Advanced LevelHubbak KhanNo ratings yet
- Clean G 315Document1 pageClean G 315bbbmxNo ratings yet
- Vacuum BoxDocument2 pagesVacuum BoxSyed Mahmud Habibur RahmanNo ratings yet
- Jee Adv - Phase 1 Paper1Document11 pagesJee Adv - Phase 1 Paper1Rebanta Bera0% (1)
- Mathworksheet of STD 5Document3 pagesMathworksheet of STD 5rameshdorage12No ratings yet
- 780USA INDEXcE1 PDFDocument21 pages780USA INDEXcE1 PDFThatchNo ratings yet
- Technical English MeasurementsDocument9 pagesTechnical English MeasurementsAndrea FieldsNo ratings yet
- NAAQSManual Volume IIDocument54 pagesNAAQSManual Volume IIdhar.kolliNo ratings yet
- AIIMS Solved Paper 1998Document23 pagesAIIMS Solved Paper 1998Débàshis DashNo ratings yet
- Draft Spec No 0-41-00-04 (Rev 03) Oct 2011Document10 pagesDraft Spec No 0-41-00-04 (Rev 03) Oct 2011Thuong NguyenNo ratings yet
- Mobrey: Boiler Water Level ControlsDocument12 pagesMobrey: Boiler Water Level ControlsMurrali Raj JeyagapalNo ratings yet
- 10BR60V2Document2 pages10BR60V2bk1313No ratings yet
- Unit1 NotesDocument56 pagesUnit1 NotesManikandanNo ratings yet
- (Frank B. Salisbury) Units Symbols and Terminolo PDFDocument249 pages(Frank B. Salisbury) Units Symbols and Terminolo PDFTrianita Amnina RosariNo ratings yet
- Problems On Basic Properties and UnitsDocument1 pageProblems On Basic Properties and UnitsJr Olivarez100% (1)
- Quantum Physics - A Beginner - S GuideDocument235 pagesQuantum Physics - A Beginner - S Guidedavid_lobos_20100% (2)
- Gravitational Potential Energy PowerpointDocument14 pagesGravitational Potential Energy Powerpointclash3355nicknameNo ratings yet
- Canon CXDI-40G X-Ray - Service ManualDocument189 pagesCanon CXDI-40G X-Ray - Service ManualLuis Fernando Garcia SNo ratings yet
- Theory - Laws of Motion+Friction - JM+JA - Live 2Document169 pagesTheory - Laws of Motion+Friction - JM+JA - Live 2bqfhwdmdcyNo ratings yet
- Scientific NotationDocument8 pagesScientific NotationrameshbalajivNo ratings yet
- International Standard I I 127-3Document7 pagesInternational Standard I I 127-3Mohammad ShamimNo ratings yet
- Mass, Weight and DensityDocument19 pagesMass, Weight and DensityKiron SheiqNo ratings yet
- Catalog Yellow JacketDocument28 pagesCatalog Yellow JacketJohn SuarezNo ratings yet
- MPE 103 - Thermodynamics First YearDocument146 pagesMPE 103 - Thermodynamics First Year3bdo MahmoudNo ratings yet
- MELC 1 ADocument33 pagesMELC 1 ANorlie LamisNo ratings yet
- Conceptual Physics Activity TheWeightDocument2 pagesConceptual Physics Activity TheWeightEdna GomezNo ratings yet
- Physical Quantity Notes Plus Mcqs-5Document19 pagesPhysical Quantity Notes Plus Mcqs-5Abdul Qadeer MughalNo ratings yet
- GENERAL CHEMISTRY For First Years of Faculties of Science, Medicine and Pharmacy Part 1Document102 pagesGENERAL CHEMISTRY For First Years of Faculties of Science, Medicine and Pharmacy Part 1Abeer TamimiNo ratings yet
- Chem 1 Problem SetDocument6 pagesChem 1 Problem SetserenatsayNo ratings yet