Professional Documents
Culture Documents
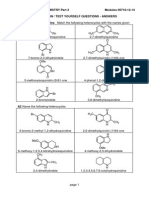
Heterocycles - PART 5 - Isoxazoles and Pyrazoles
Uploaded by
Ghadeer M Hassan0 ratings0% found this document useful (0 votes)
1 views4 pagest
Copyright
© © All Rights Reserved
Available Formats
PPTX, PDF or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this Documentt
Copyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF or read online from Scribd
0 ratings0% found this document useful (0 votes)
1 views4 pagesHeterocycles - PART 5 - Isoxazoles and Pyrazoles
Uploaded by
Ghadeer M Hassant
Copyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF or read online from Scribd
You are on page 1of 4
Heterocycles from 1,3-dicarbonyls: 8a
isoxazoles and pyrazoles
Retrosynthetic analysis
R HOR HR
R R R R
mA OR ry
HN—N oO °
a pyrazole
+ Hj,N—NH2,
R H oR HR
“yt = Wie = 1
O-N (7 Oo 0
an isoxazole + HO—NH2
Synthesis of 3,5-dimethylisoxazole and 3,5-dimethylpyrazole from pentane-2-,4-dione
NOH NH,-NH3
<—o ————_
oe 2,4 ro
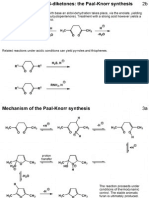
Mechanism for isoxazole / pyrazole synthesis 8b
The chemistry involved here is very similar to the Paal-Knorr pyrrole synthesis.
oO oO
HO
CH;
H3C: 7 —=
fa N
Ve
proton
{} tanstor
of
H~g
CH;
Hc y ——
X—N
:NH)XH ©nnxn
He te HNN ts
oO OW oO
ou ~H
proton {}
transfer
H
‘NXH
sy oS Hs
=> —— ©
HC UN Hye OS
3) HX~ 3) \"
compare this intermediate with the
first enol in the Paal-Knorr (slide 3a)
H
mono —— He CH
\ J |
an XN
isoxazole X
pyrazole X
Isoxazoles and pyrazoles: Unsymmetrical 1,3-diketones 9a
The reaction of unsymmetrical 1,3-diketones with hydroxylamine (NH,OH) may lead to mixtures of
isoxzoles, but with hydrazine (NHjNHz) this is not an issue as the two "products" are in fact tautomers.
Rt R? NH>-OH
——>
0 0
Rt R? NH>-NH2,
——_—
o 0
N-alkyl or aryl subsitutued hydrazines can give two products which cannot tautomerise, and so the
same question of selectivity seen with hydroxylamine arises (above)
“NA Me-NH-NH2
—
oO O
Examples of isoxazoles and pyrazoles made from 9b
unsymmetrical 1,3-diketones
The reaction of unsymmetrical 1,3 diketones may however lead to single isoxzoles or pyrazoles if one
carbonyl group is more reactive than the other.
starting material intermediate product 0
A
Cay CO2Et —-Me-NH,-NH, -H,0 \
0 Oo !
Me
Me Me,
NH,-OH
Pm i -H,0 } 1,
0 0 Ph So”
Ar = 4-NO,CgH,- Ar
Ph. Ar Me-NH,-NH2 -H,0 i
Oo ——_> ae N
0 0 N
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (894)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (587)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (119)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2219)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Adrenocorticoids2- ادوية نظري - د.احسانDocument4 pagesAdrenocorticoids2- ادوية نظري - د.احسانGhadeer M HassanNo ratings yet
- Year 2 Seminars, 06524, Semester 2, 2007 Bifunctional and Heterocyclic Chemistry TestDocument5 pagesYear 2 Seminars, 06524, Semester 2, 2007 Bifunctional and Heterocyclic Chemistry TestGhadeer M HassanNo ratings yet
- Quinoline SynthesisDocument6 pagesQuinoline SynthesisFrancesco TutinoNo ratings yet
- Acs سريرية نظري- د.حيدرDocument56 pagesAcs سريرية نظري- د.حيدرGhadeer M HassanNo ratings yet
- PK Modeling Approaches for Drug DistributionDocument67 pagesPK Modeling Approaches for Drug DistributionGhadeer M HassanNo ratings yet
- Write Your Answers On This Sheet: N O O O O O PHDocument2 pagesWrite Your Answers On This Sheet: N O O O O O PHGhadeer M HassanNo ratings yet
- 2004 OrgSeminarTestAnswerDocument2 pages2004 OrgSeminarTestAnswerGhadeer M HassanNo ratings yet
- Chapter 12 - Heterocyclic CompoundsDocument15 pagesChapter 12 - Heterocyclic CompoundsGhadeer M HassanNo ratings yet
- Cycloadditions Problems 2013Document8 pagesCycloadditions Problems 2013Ghadeer M HassanNo ratings yet
- Cyclisations Problems 2013Document6 pagesCyclisations Problems 2013Ghadeer M Hassan100% (1)
- Cycloadditions Solutions 2013 2Document9 pagesCycloadditions Solutions 2013 2Ghadeer M HassanNo ratings yet
- Cyclisations Solutions 2013Document7 pagesCyclisations Solutions 2013Ghadeer M HassanNo ratings yet
- ThiopheneDocument398 pagesThiopheneAshwin MandaleNo ratings yet
- Indole and Benzimidiazole NucleusDocument32 pagesIndole and Benzimidiazole NucleusSrikanth MalipatelNo ratings yet
- Heterocycles - PART 2 - Pall Knorr PyrroleDocument4 pagesHeterocycles - PART 2 - Pall Knorr PyrroleGhadeer M HassanNo ratings yet
- Chapter 2Document39 pagesChapter 2Ghadeer M HassanNo ratings yet
- Heterocyclic Chemistry: There Are 9 Parts To These "Animated" Notes: 2. There Are Guidance Notes and ProblemsDocument4 pagesHeterocyclic Chemistry: There Are 9 Parts To These "Animated" Notes: 2. There Are Guidance Notes and ProblemsGhadeer M HassanNo ratings yet
- Heterocycles - PART 3 - PyridazinesDocument3 pagesHeterocycles - PART 3 - PyridazinesGhadeer M HassanNo ratings yet
- Heterocycles - PART 5 - Isoxazoles and PyrazolesDocument4 pagesHeterocycles - PART 5 - Isoxazoles and PyrazolesGhadeer M HassanNo ratings yet
- Reactivity QuinolineDocument107 pagesReactivity QuinolineIan Otto100% (1)
- Heterocycles - PART 3 - PyridazinesDocument3 pagesHeterocycles - PART 3 - PyridazinesGhadeer M HassanNo ratings yet
- 2012 Hetero Model AnswerDocument3 pages2012 Hetero Model AnswerGhadeer M HassanNo ratings yet
- Heterocycles - PART 6 - PyrimidinesDocument3 pagesHeterocycles - PART 6 - PyrimidinesGhadeer M HassanNo ratings yet
- Heterocycles - PART 3 - PyridazinesDocument3 pagesHeterocycles - PART 3 - PyridazinesGhadeer M HassanNo ratings yet
- Heterocycles - PART 6 - PyrimidinesDocument3 pagesHeterocycles - PART 6 - PyrimidinesGhadeer M HassanNo ratings yet
- 2013 Hetero Model AnswerDocument2 pages2013 Hetero Model AnswerGhadeer M HassanNo ratings yet
- 2012 Hetero Model AnswerDocument3 pages2012 Hetero Model AnswerGhadeer M HassanNo ratings yet
- Year 2 Seminars, 06524, Semester 2, 2009 Heterocyclic Chemistry TestDocument4 pagesYear 2 Seminars, 06524, Semester 2, 2009 Heterocyclic Chemistry TestGhadeer M HassanNo ratings yet