Professional Documents
Culture Documents
Year 2 Seminars, 06524, Semester 2, 2009 Heterocyclic Chemistry Test
Uploaded by
Ghadeer M HassanOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Year 2 Seminars, 06524, Semester 2, 2009 Heterocyclic Chemistry Test
Uploaded by
Ghadeer M HassanCopyright:
Available Formats
YOUR STUDENT NUMBER:
Year 2 Seminars, 06524, Semester 2, 2009
Heterocyclic Chemistry Test
1.
Answer all parts.
(a)
For each of the following pairs of molecules, predict and explain which is the more
basic.
(i)
N
H
Basic
N
H2
Cl
Non basic
no non-bonded (lone)
pair of electrons
N
H2
less conjugated
more conjugated
(ii)
N
N
Me
Me
less stable conjugate base more stable conjugate base
more basic
less basic
H
N
Me
N
Me
(PTO)
(b)
Which of the following molecules are aromatic, anti-aromatic or simply conjugated?
BH
O
Cl
Na
Cyclic - YES
Cyclic - YES
Cyclic - YES
Planar - YES
Planar - YES
Planar - YES
Continuous conjugation - YES
Continuous conjugation - YES
Continuous conjugation - YES
Number of pi-electrons = 6
Number of pi-electrons = 6
Number of pi-electrons = 6
AROMATIC (4n+2) n = 1
AROMATIC (4n+2) n = 1
AROMATIC (4n+2) n = 1
(PTO)
YOUR STUDENT NUMBER:
Year 2 Seminars, 06524, Semester 2, 2009
Bifunctional Chemistry Test
2.
Answer all parts.
(a)
Mark on the following diagram all of the acidic hydrogen atoms in compound A and
indicate clearly, with a brief explanation, which is the most acidic.
O
EtO2C
H
CN
most acidic:
enolate conjugation extends
over C N too.
O
A
(b)
Draw a tautomeric form of the compound B.
H
NH
N
H
less stable: loss of
aromaticity
B
(c)
tautomer
For each of the following (see overleaf) work out what carbonyl-containing
compounds could condense to form the molecules shown. In each case, giving a
reason, state whether a synthesis from your two starting materials would be
practicable (i.e. the reaction would give you the target molecule as the sole product).
(PTO)
Br
(i)
Br
O +
Br
Br
O
H
Br
2x
NaOH
EtOH
O +
Br
ALDOL crossed
condensation
Br
O
H
O
Only ketone can enolize, aldehyde is more reactive electrophile than ketone: crossed condensation
practicable. (This is essentially the same as question 1 tutorial sheet 4, and the dibenzylidene acetone
lab experiment).
Br
Br
(ii)
O +
Br
Br
O
2x
O
Br
O +
Br
ALDOL crossed
condensation
NaOH
EtOH
Br
O
Both ketones can enolize, both have similar reactivity as electrophile: crossed condensation NOT
practicable. Mixture of self and crossed condensation products obtained. (This is similar to question
2 tutorial sheet 4)
(iii)
EtO
EtO
O
2x
+ EtO
O
O
NaOEt
EtOH
EtO
O
EtO
O
+ EtO
O
EtO
O
CLAISEN condensation comes from 2 moles of the same ester: condensation practicable.
(This is essentially the same as question 1 tutorial sheet 6)
(END)
You might also like
- Ch1b Ps3 Key SerDocument7 pagesCh1b Ps3 Key SerRichard ZhuNo ratings yet
- Chemistry 303 Final Exam KeyDocument22 pagesChemistry 303 Final Exam KeyaegaisNo ratings yet
- C331 S02 Test 1Document7 pagesC331 S02 Test 1api-19827675No ratings yet
- Aromatics HandoutDocument8 pagesAromatics HandoutJan ChretienNo ratings yet
- CHEM 222 Chap 1 HW F21 KEYDocument3 pagesCHEM 222 Chap 1 HW F21 KEYJessicaNo ratings yet
- Basic Chemistry Review Packet Key EL 15-16Document6 pagesBasic Chemistry Review Packet Key EL 15-16Shayla MaysNo ratings yet
- Pentadienyl CationDocument7 pagesPentadienyl CationAbhishek SardaNo ratings yet
- Aromatic ChemistryDocument16 pagesAromatic ChemistrysimbaleoNo ratings yet
- KIM 101E - Week 3 - BDocument70 pagesKIM 101E - Week 3 - Baliyasin200000No ratings yet
- Chemistry - Concepts and Multiple ChoiceDocument5 pagesChemistry - Concepts and Multiple ChoiceGeorge Isaac McQuiles100% (1)
- Do Not Open This Test Until Everyone Has OneDocument12 pagesDo Not Open This Test Until Everyone Has OneChemist MeNo ratings yet
- Assignment 4 Reactions of Aromatic Compounds AnswersDocument11 pagesAssignment 4 Reactions of Aromatic Compounds AnswersJonathan Yeung100% (1)
- O14 AromaticDocument11 pagesO14 AromaticDottie Landreth BaileyNo ratings yet
- SamRobinson Lecture Notes 5Document25 pagesSamRobinson Lecture Notes 5Pigeon BNo ratings yet
- Organic Chemistry Practice Questions on Alkenes and HalidesDocument4 pagesOrganic Chemistry Practice Questions on Alkenes and Halidessowmmiya karuppiahNo ratings yet
- 303 99 1stExKEY PDFDocument8 pages303 99 1stExKEY PDFaegaisNo ratings yet
- Benzene and Aromatic Compounds-fazli-In ClassDocument57 pagesBenzene and Aromatic Compounds-fazli-In Classjokowi123No ratings yet
- Introduction To Organic Chemistry Ii CHEM 224: Answer KeyDocument8 pagesIntroduction To Organic Chemistry Ii CHEM 224: Answer Keygautamtajesh1983No ratings yet
- Tutorial Letter 201/2/2017: General Chemistry 1BDocument16 pagesTutorial Letter 201/2/2017: General Chemistry 1BLeigh MakanNo ratings yet
- Why Bother With Organic Synthesis?: Chemists Need To Make Them!Document34 pagesWhy Bother With Organic Synthesis?: Chemists Need To Make Them!FarhanAkramNo ratings yet
- CH1O3 Questions PDFDocument52 pagesCH1O3 Questions PDFPrince T MashandaNo ratings yet
- Week 3 Study Groups WorksheetDocument5 pagesWeek 3 Study Groups WorksheetreallechuaNo ratings yet
- OchempractwrkshtDocument13 pagesOchempractwrkshtlabaileyNo ratings yet
- Unit 3 Chemical Equations and Hydrate Lab ReportDocument10 pagesUnit 3 Chemical Equations and Hydrate Lab ReportSophie DanhNo ratings yet
- A. 50 B. 30 C. 15 D. 5: 5.12 Practice Exam #4 PAGE 1 Short Questions 1-8 3 Points Each)Document7 pagesA. 50 B. 30 C. 15 D. 5: 5.12 Practice Exam #4 PAGE 1 Short Questions 1-8 3 Points Each)Armando Shehi SayhellotogoodbyeNo ratings yet
- Selected Questions of Chapter Aldehyde K Solved Sample Papers For Class 12 ChemistryDocument33 pagesSelected Questions of Chapter Aldehyde K Solved Sample Papers For Class 12 ChemistrySsNo ratings yet
- CHM 332 Fourth Examination NotesDocument7 pagesCHM 332 Fourth Examination NoteskanilkadianNo ratings yet
- 231 F 2010 Practice MT4 - Pp1to12Document12 pages231 F 2010 Practice MT4 - Pp1to12Chemist MeNo ratings yet
- Organic ChemistryDocument2 pagesOrganic ChemistryRacheal KirbyNo ratings yet
- Test Bank For Organic Chemistry 9Th Edition Mcmurry 1305080483 9781305080485 Full Chapter PDFDocument18 pagesTest Bank For Organic Chemistry 9Th Edition Mcmurry 1305080483 9781305080485 Full Chapter PDFclarence.kuhns728100% (11)
- Guess Paper 3 Isc Chemistry (1)Document5 pagesGuess Paper 3 Isc Chemistry (1)SanjanaNo ratings yet
- CH 231 Old Exams 1 and 2Document13 pagesCH 231 Old Exams 1 and 2Brian DaSilva100% (3)
- Revision Worksheet For Half Yearly Exam Chemistry From Basic Principles of Organic ChemistryDocument2 pagesRevision Worksheet For Half Yearly Exam Chemistry From Basic Principles of Organic ChemistryVrisanNo ratings yet
- Student Exploration: Chemical EquationsDocument6 pagesStudent Exploration: Chemical EquationsAndreNo ratings yet
- Chemistry Set-1 BSSC Term IDocument10 pagesChemistry Set-1 BSSC Term IKavin SatyaNo ratings yet
- CHM 1321 Assignment 4 AnswersDocument12 pagesCHM 1321 Assignment 4 AnswersSara YuenNo ratings yet
- 2008 Promo 1Document15 pages2008 Promo 1shinkir0No ratings yet
- OrganicDocument6 pagesOrganicTrent SwordsNo ratings yet
- H2 Paper 1 Answers 2011 PDFDocument9 pagesH2 Paper 1 Answers 2011 PDFNg Wei BinNo ratings yet
- Chemistry Concepts and Multiple Choice PDFDocument5 pagesChemistry Concepts and Multiple Choice PDFHandugan Quinlog NoelNo ratings yet
- Organic Exam Answer.Document11 pagesOrganic Exam Answer.S JNo ratings yet
- CHM 1321 Assignment #2 - : AnswersDocument11 pagesCHM 1321 Assignment #2 - : AnswersSara YuenNo ratings yet
- Answer Key: Chemistry 206 First Hour ExaminationDocument9 pagesAnswer Key: Chemistry 206 First Hour Examinationsudipta88No ratings yet
- Write Only in The Space Provided For Each Question. Score: I - /31 II - /12 III - /28 IV - /11 V - /05 VI - /05 VII - /08 Total: /100Document15 pagesWrite Only in The Space Provided For Each Question. Score: I - /31 II - /12 III - /28 IV - /11 V - /05 VI - /05 VII - /08 Total: /100aegaisNo ratings yet
- Gen Chem. Module-7Document29 pagesGen Chem. Module-7dzai leigh75% (4)
- Chemistry 108B Exam #1 reagentsDocument1 pageChemistry 108B Exam #1 reagentsNorma Leticia RamosNo ratings yet
- Aromatic Compounds - Chapter 14 Is Mostly Descriptive and Does NotDocument9 pagesAromatic Compounds - Chapter 14 Is Mostly Descriptive and Does NotRafid InamNo ratings yet
- Chemistry SGTA WEEK 3Document3 pagesChemistry SGTA WEEK 3kassy jayNo ratings yet
- 222-10 Exam #1 Answers PDFDocument8 pages222-10 Exam #1 Answers PDFbrich22No ratings yet
- Sample - CHEMISTRYDocument5 pagesSample - CHEMISTRYShiella Mae Baltazar BulauitanNo ratings yet
- Chemistry SQPDocument4 pagesChemistry SQPstressNo ratings yet
- Test - 2 - Solutions and FeedbackDocument7 pagesTest - 2 - Solutions and FeedbackNondumiso MavundlaNo ratings yet
- Organic Chemistry Test 1 MemorandumDocument7 pagesOrganic Chemistry Test 1 MemorandumSandile SynthaxError Mabika0% (1)
- Chapter 1 Fundamentals of Organic ChemistryDocument5 pagesChapter 1 Fundamentals of Organic ChemistryOchem90No ratings yet
- CL-XI SC-Half Yearly-2021 (CHEM)Document5 pagesCL-XI SC-Half Yearly-2021 (CHEM)Rapelly NagarajuNo ratings yet
- Intermolecular Forces Lab Report WorksheetDocument5 pagesIntermolecular Forces Lab Report WorksheetSam RobertsNo ratings yet
- Examen Campinas InglesDocument7 pagesExamen Campinas InglesSharon Laurente RamónNo ratings yet
- 303 09FinalKEY PDFDocument15 pages303 09FinalKEY PDFaegaisNo ratings yet
- Practice Makes Perfect in Chemistry: Compounds, Reactions and Moles with AnswersFrom EverandPractice Makes Perfect in Chemistry: Compounds, Reactions and Moles with AnswersRating: 3 out of 5 stars3/5 (2)
- Schaum's Easy Outline of Organic Chemistry, Second EditionFrom EverandSchaum's Easy Outline of Organic Chemistry, Second EditionRating: 3.5 out of 5 stars3.5/5 (2)
- Quinoline SynthesisDocument6 pagesQuinoline SynthesisFrancesco TutinoNo ratings yet
- Acs سريرية نظري- د.حيدرDocument56 pagesAcs سريرية نظري- د.حيدرGhadeer M HassanNo ratings yet
- PK Modeling Approaches for Drug DistributionDocument67 pagesPK Modeling Approaches for Drug DistributionGhadeer M HassanNo ratings yet
- Adrenocorticoids2- ادوية نظري - د.احسانDocument4 pagesAdrenocorticoids2- ادوية نظري - د.احسانGhadeer M HassanNo ratings yet
- Year 2 Seminars, 06524, Semester 2, 2007 Bifunctional and Heterocyclic Chemistry TestDocument5 pagesYear 2 Seminars, 06524, Semester 2, 2007 Bifunctional and Heterocyclic Chemistry TestGhadeer M HassanNo ratings yet
- Indole and Benzimidiazole NucleusDocument32 pagesIndole and Benzimidiazole NucleusSrikanth MalipatelNo ratings yet
- 2004 OrgSeminarTestAnswerDocument2 pages2004 OrgSeminarTestAnswerGhadeer M HassanNo ratings yet
- Write Your Answers On This Sheet: N O O O O O PHDocument2 pagesWrite Your Answers On This Sheet: N O O O O O PHGhadeer M HassanNo ratings yet
- ThiopheneDocument398 pagesThiopheneAshwin MandaleNo ratings yet
- Heterocyclic Chemistry: There Are 9 Parts To These "Animated" Notes: 2. There Are Guidance Notes and ProblemsDocument4 pagesHeterocyclic Chemistry: There Are 9 Parts To These "Animated" Notes: 2. There Are Guidance Notes and ProblemsGhadeer M HassanNo ratings yet
- Chapter 12 - Heterocyclic CompoundsDocument15 pagesChapter 12 - Heterocyclic CompoundsGhadeer M HassanNo ratings yet
- Chapter 2Document39 pagesChapter 2Ghadeer M HassanNo ratings yet
- Cyclisations Problems 2013Document6 pagesCyclisations Problems 2013Ghadeer M Hassan100% (1)
- Cyclisations Solutions 2013Document7 pagesCyclisations Solutions 2013Ghadeer M HassanNo ratings yet
- Cycloadditions Solutions 2013 2Document9 pagesCycloadditions Solutions 2013 2Ghadeer M HassanNo ratings yet
- Cycloadditions Problems 2013Document8 pagesCycloadditions Problems 2013Ghadeer M HassanNo ratings yet
- Reactivity QuinolineDocument107 pagesReactivity QuinolineIan Otto100% (1)
- Heterocycles - PART 6 - PyrimidinesDocument3 pagesHeterocycles - PART 6 - PyrimidinesGhadeer M HassanNo ratings yet
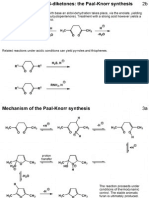
- Heterocycles - PART 2 - Pall Knorr PyrroleDocument4 pagesHeterocycles - PART 2 - Pall Knorr PyrroleGhadeer M HassanNo ratings yet
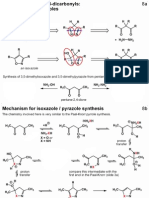
- Heterocycles - PART 5 - Isoxazoles and PyrazolesDocument4 pagesHeterocycles - PART 5 - Isoxazoles and PyrazolesGhadeer M HassanNo ratings yet
- Heterocycles - PART 3 - PyridazinesDocument3 pagesHeterocycles - PART 3 - PyridazinesGhadeer M HassanNo ratings yet
- Heterocycles - PART 3 - PyridazinesDocument3 pagesHeterocycles - PART 3 - PyridazinesGhadeer M HassanNo ratings yet
- 2012 Hetero Model AnswerDocument3 pages2012 Hetero Model AnswerGhadeer M HassanNo ratings yet
- Heterocycles - PART 5 - Isoxazoles and PyrazolesDocument4 pagesHeterocycles - PART 5 - Isoxazoles and PyrazolesGhadeer M HassanNo ratings yet
- Heterocycles - PART 6 - PyrimidinesDocument3 pagesHeterocycles - PART 6 - PyrimidinesGhadeer M HassanNo ratings yet
- Heterocycles - PART 3 - PyridazinesDocument3 pagesHeterocycles - PART 3 - PyridazinesGhadeer M HassanNo ratings yet
- 2013 Hetero Model AnswerDocument2 pages2013 Hetero Model AnswerGhadeer M HassanNo ratings yet
- 2012 Hetero Model AnswerDocument3 pages2012 Hetero Model AnswerGhadeer M HassanNo ratings yet
- Jyothish Kumar Took Sulphur Powder On A Spatula and Heated It. HeDocument9 pagesJyothish Kumar Took Sulphur Powder On A Spatula and Heated It. HeSharan SiuuNo ratings yet
- NTU - Qualitative Analysis of Cation Group 1Document7 pagesNTU - Qualitative Analysis of Cation Group 1Alondra CuevasNo ratings yet
- Swot Analysis: StrengthDocument4 pagesSwot Analysis: StrengthDee DeeNo ratings yet
- Earth and Life ScienceDocument30 pagesEarth and Life ScienceChristian VitalesNo ratings yet
- Cutback Bitumen MC 3000Document2 pagesCutback Bitumen MC 3000muhirwa raymondNo ratings yet
- Above & Beyond: Compressed Air & Gas FiltrationDocument4 pagesAbove & Beyond: Compressed Air & Gas FiltrationPaul GirkinNo ratings yet
- Engineering Encyclopedia: Determining Acceptability of Materials For Storage TanksDocument25 pagesEngineering Encyclopedia: Determining Acceptability of Materials For Storage TanksAfzaalUmairNo ratings yet
- Some Basic Concept of ChemistryDocument81 pagesSome Basic Concept of ChemistryZaid KhanNo ratings yet
- Mechanism of PolymeristionDocument25 pagesMechanism of PolymeristionAshish Kumar SinghNo ratings yet
- Acid Base Titration Experiment 4Document10 pagesAcid Base Titration Experiment 4pokesurfer100% (3)
- 3RD TERM S 1 - ChemistryDocument30 pages3RD TERM S 1 - ChemistryAdelowo DanielNo ratings yet
- Ceramic Coating Product Data SheetDocument3 pagesCeramic Coating Product Data SheetANIBALLOPEZVEGANo ratings yet
- Bio-Electricity Generation From Sugar Mill Waste WDocument3 pagesBio-Electricity Generation From Sugar Mill Waste WKavi GowdaNo ratings yet
- Cellulose Crystallinity Index Measurement Techniques and Their Impact On Interpreting Cellulase PerformanceDocument10 pagesCellulose Crystallinity Index Measurement Techniques and Their Impact On Interpreting Cellulase PerformanceParvinder McCartneyNo ratings yet
- Liquid preparations for cutaneous careDocument3 pagesLiquid preparations for cutaneous careAna MariaNo ratings yet
- Fiche Technique Pigement Noir Orion Oec - 3065 - r7 - Carbon - Black - Pigments - Tech - Data - v2 - 5 - 10 - 19Document8 pagesFiche Technique Pigement Noir Orion Oec - 3065 - r7 - Carbon - Black - Pigments - Tech - Data - v2 - 5 - 10 - 19anisov34No ratings yet
- Prescription WritingDocument28 pagesPrescription WritingEshana AryaNo ratings yet
- Characterisation of Fluorescence Background in Dye TracingDocument7 pagesCharacterisation of Fluorescence Background in Dye TracingALCIRA VALERIA CÉSPEDES VARGASNo ratings yet
- ME198 Main Engine Cylinder Liner TroubleDocument2 pagesME198 Main Engine Cylinder Liner TroubleRani NoumanNo ratings yet
- Master Catalog PetroDocument76 pagesMaster Catalog PetroAlexSNo ratings yet
- Pengolahan Air Boiler: OlehDocument35 pagesPengolahan Air Boiler: OlehPuput MawartiNo ratings yet
- Worksheet-Nernst Equation PDFDocument4 pagesWorksheet-Nernst Equation PDFLedd SleddNo ratings yet
- % Chapter 27: Molecular GeneticsDocument39 pages% Chapter 27: Molecular Geneticsdds uwuNo ratings yet
- Galozinc 1180Document48 pagesGalozinc 1180Pramod SherkarNo ratings yet
- Nanozymes TechnologyDocument10 pagesNanozymes TechnologyAfiqah FarzanaNo ratings yet
- BS 60079-10-2Document30 pagesBS 60079-10-2Ade KalejaiyeNo ratings yet
- PRC-II Lab ManualDocument41 pagesPRC-II Lab ManualRana Asad AliNo ratings yet
- Form 5 Biology Notes C1 & C2Document18 pagesForm 5 Biology Notes C1 & C2m-7940668No ratings yet
- Kathonlx1 5%TDSDocument9 pagesKathonlx1 5%TDSPablo Manu Gomez VNo ratings yet
- Springer Proceedings in PhysicsDocument482 pagesSpringer Proceedings in PhysicsbazediNo ratings yet