Professional Documents
Culture Documents
Jesse Wackerbarth - CNS Pathogens Revised
Uploaded by
MicroposterOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Jesse Wackerbarth - CNS Pathogens Revised
Uploaded by
MicroposterCopyright:
Available Formats
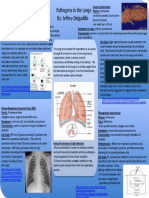
Herpes Simplex Encephalitis (HSE) Streptococcus pneumoniae: Bacterial Meningitis
HSE is a rare infection of the brain by the Herpes simplex virus-1 (HSV-
Pathogenic Streptococcus pneumoniae is a gram positive, diplococci shaped
1), the causative pathogen of common orofacial cold sores. Infection bacteria of the phylum firmicutes. It is an extracellular pathogen
causes severe necrotizing encephalitis (particularly in the temporal
lobes, where small hemorrhages may occur). Classic encephalitic Infections and one of the most prevalent and serious causes of bacterial
meningitis in humans, proving fatal for 30% of patients and causing
long-term neural sequelae in around 40% of survivors.
symptoms including confusion, altered mental status, personality
changes, fever, and potentially seizures. The condition is extremely
dangerous to the fragile CNS and 70% untreated cases will be fatal; of
those treated a third will die and only 20% will recover without long
of the CNS Streptococcus pneumoniae (or pneumococcus) is part of the
normal flora observed in the human upper respiratory tract, but can
opportunistically cause disease under the right conditions.
term neuro-cognitive damage. HSV-1 is a member of the Jesse Wackerbarth
Herpesviridae and is a relatively large, double stranded DNA, Breaking the Wall: The Trojan Horse
enveloped virus. It is spread primarily by direct contact with a localized Pneumococcus colonizes the nasopharynx, then invades the
Above Right: Major CNS complications secondary to acute bacterial intravascular space. Once in the bloodstream, pneumocuccus
infected areas (classically cold sores) though there can still be some
Above: Severe Encephalitis cause by meningitis. (A) Brain edema. (B) Hydrocephalus. (C) Cerebral vasculitis with evades the immune system with its thick polysaccharide capsule, a
shedding of viral particles in the absence of a symptomatic
HSE, particularly in left temporal lobe, multiple cerebral infarctions. (D) Sinus thrombosis with venous infarction major virulence factor that cloaks the bacteria’s antigenic surfaces
manifestation.
visualized via MRI. and mild cerebral hemorrhage (black arrow). and provides a strong anti-phagocytic advantage. Additionally, the
capsule provides protection from the attacks of complement and
Breaking the Wall: The secret tunnel CNS Immune Privilege the production of antibodies. With a high bacterial load in the
The billions of Neurons that constitute the CNS, allowing us to think, move, breath, and live, bloodstream CNS infiltration may occur. The exact site of entry into
HSV gains cellular entry by envelope fusion with target membranes based on glycolipid-recetor interactions. Once
are special; unlike most other cells in the body, they cannot divide or be effectively the CSF remains unclear and highly debated. The general strategy
inside, HSV-1 capsid travels to the cell nucleus, where it injects its genome through a portal generated by UL6
repaired, the neurons we have are irreplaceable. While the classic immune response is involves first attaching to epithelial cells at several glycoconjugates.
proteins. The complete virus is assembled in the nucleus and, eventually, through a complex pathway of nuclear
essential and effective in most of the body, its veracity often leads to extensive localized They then activate the host epithelial cells to increase the
budding, replicated virions are excytosed from the host cell.
tissue damage from our own immune cells. For most physiological systems this is necessary expression of surface platelet-activating factor (PAF) receptor,
HSV-1 has the potential for latency, in which viral proteins of the lytic cycle are not produced and the virus lies Above: Pneumococcus’s basic path to the
and generally reparable collateral damage, but for the CNS it can be devastating and which binds to phosphorylcholine in the bacterial cell wall. When
dormant, particularly in the sensory neural ganglia. It is currently speculated that from this neuro-infective CNS, Below: Activation of immune response.
irreversible. The rigid skull and spinal column leaves very little room for inflammation bound, PAF receptors are coded to endocytose, in this case bringing
capability, through unknown mechanisms and activations, HSV-1 virus gains entry to the peripheral neural system
without dangerous consequences. Thus it is necessary that the CNS be immunologically along the attached pneumococcus into the interior of the cell.
and migrates through the peripheral axons to infect the CNS (via retrograde axonal flow), thereby avoiding the
privileged, distinct from the peripheral immune system and more delicate in its Though some will perish within the host cell, the bacteria (though
formidable BB barrier. It remains unclear precisely how and where this infiltration occurs, though current research
responses, though their interactions are numerous and complex. Immune privileged areas not an intercellular pathogen) can travel through the epithelial
has implicated the olfactory nerve as a likely candidate.
are characterized partly by a greater tolerance to potential antigen, such as transplanted cell, and emerge to infect the CNS.
Causing Disease
Pathologically, HSV infected cells can balloon in size, degrading the plasma membrane and nuclear structure into tissues or foreign organisms, but also by how they mount a response when necessary; thus
the CNS is not immune-deficient, but highly immune-specialized. Causing Disease
multi nucleated large cells. The virus elicits a strong immune response, engaging first the microglia, which up-
Once inside the CNS, microglia respond to infection but are even
regulate their activity and expression of antigen presenting MHC proteins, and leads to increased infiltration of Great Wall of CNS: The Blood Brain Barrier more inept in their phagocytic activity than the peripheral immune
granulocytes and t lymphocytes to the site of infection. HSV-1 has been demonstrated to productively infect both The CNS is separated from blood stream (and much of the generalized immune system) by cells. Interestingly, phase variation, in which CNS invasive
neurons and astrocytes, but seems to have little productive infectious capability in microglia. Microglia however the blood-brain barrier, a system of electrically resistant tight junctions linking the epithelial pneumococcus express higher levels of teichoic acid and cell wall
have been show to induce apoptosis and release large quantities of neurotoxic cytokines when infected even cells that line the capillaries providing the brain’s blood supply. This cellular barrier allows proteins relative to capsule, seems to heighten the CNS response.
nonproductively. Thus damage to the CNS tissue is thought to be partly from the infectious activities of the virus, gas diffusion and nutrient transfer through transport, but generally prevents the migration Typical antigenic recognition of PAMP regions and the toxin
but perhaps more critically from the wide spectrum of cytotoxic compounds and acute inflammation elicited by of large particles, both pathogenic and immune, from the blood stream into the cerebral Pneumolysin leads to a strong immune response, consisting first in
the immune response. This is evidenced in the prolonged activation of microglia for up to 12 months after spinal fluid (CSF), thereby isolating and protecting the CNS environment. The permeability the activation of microglia, releasing damaging inflammatory and
treatment with antivirals and resolution. The latency and reactivation characteristics of herpes simplex in other of the BB barrier is thought to be regulated by the activity of adjacent CNS cells called cytotoxic cytokines, and second, in the destructive recruitment of
regions of the body is not typically observed in HSE, as the retrograde axonal migration of the virus appears astrocytes. Pathogens that infect the CNS must have some mechanism for avoiding or peripheral immune cells. Both contribute to swelling and neural cell
extremely rare phenomena and is not linked to prolonged infection, as only 10% of those who develop HSE report overcoming these formidable barriers. destruction that leads to symptomatic disease and potentially
having a history of recurrent cold sores or other HSV manifestations.
Resident Immune System death; the bacteria itself, lacking the capacity for neural
Microglia are the CNS macrophages and the workhorse of the localized immune response. intracellular invasion or serious toxic production does little real
In the absence of pathogen they play a mostly neuro-supportive role, scanning for and CNS damage.
Cerebral Malaria: Plasmodium falciparum removing damaged tissues and plaques, but in times of infection they are activated as
specialized CNS immune soldiers. Microglia have many functions, including the classics: Mycobacterium tuberculosis: CNS Tuberculosis
Cerebral malaria (CM) is a serious and life-threatening complication of non-specific antigen recognition, phagocytic and cytotoxic activity, cytokine production, as
malarial disease that affects more than a million lives annually. well as antigen presentation and t cell activation in substantial infections (with expression of
Infection is primarily caused by the protozoan parasite Plasmodium MHC class I and II); great plasticity and sensitivity is necessary due to their isolated, fragile
environment—for only in cases of extreme infection are peripheral immune cells recruited Mycobacterium tuberculosis is an aerobic, acid-fast gram positive bacilli,
falciparum, which is carried and transmitted to humans by the female characterized by it slow growth and waxy cell wall with high lipid content
Anopheles mosquito. Malaria involves a complex parasitic life cycle, through a degraded BB barrier, often causing the most immune damage.
(particularly mycolic acid). This facultative intercellular pathogen is the
first reproducing in the liver then, emerging into the blood stream, causative agent of tuberculosis, a primarily respiratory disease affecting
where the parasites preferentially infect red blood cells. Malaria is an nearly a third of the world’s population, which, in around 1% of cases, can
enormous global health challenge, infecting over 250 million people progress to a rare, high mortality infection of the CNS. Left untreated, CNS
annually though only 1 to 2% will develop a neurological tuberculosis is invariably fatal. The onset of neurological symptoms
manifestation called cerebral malaria (CM), the majority of which will progresses similarly to most CNS infections, beginning with mild
be children. CM is characterized by the development of neurological complaints like headache, fever, or dizziness and progressing to severe
symptoms accompanying classic malaria infection. Victims may Above: Tubercles (white
Section of brain showing blood neurocognitive disturbances, altered mental status and seizures typical of
become delirious, confused, altered, or dizzy and can progress to spots) in the CNS can be
vessels blocked with developing P. CNS inflammation.
seizures, coma, and death. clearly visualized with
falciparum parasites (see arrows) MRI.
Breaking the Wall: The Siege Breaking the Wall: The Breach
The neuropathology of CM still not very well understood, however researchers have demonstrated that it begins with high Mycobacterium tuberculosis (MTB) first infects the human host through inhalation of respiratory droplets
levels of parasitically infected erythrocytes (red blood cells) in the blood stream. Infection of red blood cells may enhance ,preferentially infecting the alveolar macrophages through a variety of phagocytosis inducing receptors. Once
the expression of P. falciparum erythrocyte membrane protein (PfEMP-1), which binds to ligands on endothelial cells, such inside, M. tuberculosis hijacks the phagosome and proliferates within the immune cells. Classically, pulmonary
as ICAM-1 or E-selectin. As masses of infected erythrocytes adhere to the deep microvasculature serving the CNS, normal MTB infection produces a massive inflammatory response and the charteristic formation of granulomas; as
flux is occluded and the CNS interior may become progressively stressed and hypoxic, contributing to the development of the infection progresses low levels of MTB have the capacity to spread through the blood stream and
neurological symptoms and eventually coma. It has also been implicated that a malarial toxin may induce the release of Above: Intact erythrocytes surrounding a lymphatic system, occasionally colonizing the CNS. It has been speculated that MTB migrates through
capillary in the cerebral cortex. Endothelial cell protective epithelial cells independently, or within infected macrophages; however, recent research on animal
cytokines by macrophages, which leads to the uncontrolled production and accumulation of toxic nitric oxide in the CNS
layer appears to have disintegrated models indicates that MTB may not gain access to the CNS through infiltration of the blood-brain barrier, as
and, later, degradation of the BB barrier.
Causing Disease is typical of bacterial CNS invasions. Instead MTB can gain access to the subarachnoid space through the
The accumulation of infected blood cells brings a rapid host peripheral immune response, in the form of T lymphocytes and Above: Murine model of CNS tuberculosis. (A) rupture of adjacent parenchymal tubercle or a caseating vascular focus , thereby bypassing the barrier
monocytes—further crowding the already occluded capillaries. Interestingly, the CNS immune system responds as well, Coronary section at the level of the caudal defense and gaining entry to the vulnerable CNS.
probably from both environmental stress and an influx of cytokine signaling, leading to the activation of microglia. Activated diencephalon, with multifocal nonsuppurative
microglia contribute to even greater cytokine production from both sides, especially TNF-α, and result in the degradation of encephalitis. (B) Cornu ammonis showing mild Causing Disease
the epithelial blood brain barrier, with some observed migration of microglia cells. As the epithelial layer weakens, micro perivascular lymphocytic and histiocytic Once inside the CNS, M. tuberculosis effectively and productively infects microglia cells due to their
hemorrhaging can occur into the CNS bringing with it infected erythrocytes and elements of the immune response. Immune infiltration, with microgliosis and reactive mechanistic similarities to macrophages. The facilitate uptake via a variety of receptors, mainly the CD14
activation, as well as some cytotoxic production by the parasite, damages the astrocytes and decreases regulatory astroglia. (C) Dorsal third ventricle, choroid plexus, receptor when not nonopsonized. Rapid cytokine release, particularly of TNF-α, leads to inflammation and
control, stressing the CNS neural environment. Severe, late stage cases can lead to the formation of ring-like liaisons on the and subependymal areas expanded by increased permeability of the BB barrier and the rapid recruitment of destructive peripheral immune cells,
brain. lymphocytic, plasmacytic, and histiocytic classically forming disruptive tubercular granulomas throughout different areas of the CNS infection. The
infiltration, with subependymal microgliosis and immune response against intercellular pathogens and with recruited lymphocytes is exceptionally destructive
Though fatal in 30 to 40% of cases even when treated effectively with anti-malarial drugs, survivors of CM have a relatively reactive astroglia. and feeds back to an even greater inflammatory response. Depending on the area of entry MTB can cause
low risk of long term neurological impairments. Since infection does not include a large scale immune response within the either encephalitis or meningitis, and can even form brain abscesses—all of these life-threatening conditions
Above: Semi-thin section of capillary in brainstem. Enlarged perivascular space (*) contribute the severe neurological symptoms and the high mortality of the infection.
CNS or the recruitment of the often destructive peripheral immune cells, only about 10% of cases experience long-term
containing leukocytes in close vicinity to the vessel. Lymphocytes (arrows) and
symptoms or deficits.
monocyte (arrowhead) sequestered to the endothelial wall.
You might also like
- Pathogenesis Infection of CnsDocument4 pagesPathogenesis Infection of CnshadiNo ratings yet
- CNS Pathogens-Jesse WackerbarthDocument1 pageCNS Pathogens-Jesse WackerbarthMicroposterNo ratings yet
- Clinpharm Notes 4 TopicsDocument8 pagesClinpharm Notes 4 TopicsALESANDRA DAWN PAYOTNo ratings yet
- Neurologic InfectionsDocument6 pagesNeurologic InfectionsHazel ZullaNo ratings yet
- Bacterial Meningitis and Other Nonviral Infections of The Nervous SystemDocument13 pagesBacterial Meningitis and Other Nonviral Infections of The Nervous Systemgeancarla mendozaNo ratings yet
- Communicable DiseaseDocument12 pagesCommunicable DiseaseFatema Tuzzannat BrishtyNo ratings yet
- Cshperspectmed BAC A012393Document14 pagesCshperspectmed BAC A012393RaffaharianggaraNo ratings yet
- CNS - Infections F2022 OVER VIEWDocument18 pagesCNS - Infections F2022 OVER VIEWMadison MillwoodNo ratings yet
- 12 Cns Infection 2 LecturesDocument18 pages12 Cns Infection 2 LecturesZain AlAbideen AlTaeeNo ratings yet
- Managing Meningoencephalitis in Indian ICU: Neurocritical CareDocument5 pagesManaging Meningoencephalitis in Indian ICU: Neurocritical CareerikafebriyanarNo ratings yet
- Central Nervous System Infection in The PediatricDocument5 pagesCentral Nervous System Infection in The PediatricJeriz Marie GamboaNo ratings yet
- Infectious Diseases Pharmacotherapy: Lesson 5 Central Nervous System InfectionDocument63 pagesInfectious Diseases Pharmacotherapy: Lesson 5 Central Nervous System Infectionbest batiNo ratings yet
- Diseases of The Central Nervous SystemDocument5 pagesDiseases of The Central Nervous SystemWTF192No ratings yet
- Vaccines 09 00209 v2Document16 pagesVaccines 09 00209 v2Rin ChanNo ratings yet
- How Do Extracellular Pathogens Cross The Blood-Brain Barrier?Document6 pagesHow Do Extracellular Pathogens Cross The Blood-Brain Barrier?Jade LoberianoNo ratings yet
- Problem 3.01 Nervous System Study Guide 3Document2 pagesProblem 3.01 Nervous System Study Guide 3Monish NaiduNo ratings yet
- Pathology BrainDocument4 pagesPathology BrainAshuNo ratings yet
- Bacterial Infections of The Central Nervous System: Paul A. Lapenna, Do Karen L. Roos, MDDocument9 pagesBacterial Infections of The Central Nervous System: Paul A. Lapenna, Do Karen L. Roos, MDOver HidalgoNo ratings yet
- CNs InfectionDocument6 pagesCNs InfectionChefera AgaNo ratings yet
- Activity IiDocument5 pagesActivity IiYVONNE PEARL BALIQUIDNo ratings yet
- Neuro B2.1 Viral Infections of The CNSDocument10 pagesNeuro B2.1 Viral Infections of The CNSKendel Ann KempisNo ratings yet
- Pathogenesis of Bacterial Meningitis: From Bacteraemia To Neuronal InjuryDocument10 pagesPathogenesis of Bacterial Meningitis: From Bacteraemia To Neuronal InjurymjeanjNo ratings yet
- Bact and DiseaseDocument5 pagesBact and DiseasePatrick MatubayNo ratings yet
- Bacterial Meningitis and Brain Abscess: Key PointsDocument7 pagesBacterial Meningitis and Brain Abscess: Key PointsMartha OktaviaNo ratings yet
- Pathophysiology of TBDocument3 pagesPathophysiology of TBEddie Lou GuzmanNo ratings yet
- PatophysDocument1 pagePatophysbdhdNo ratings yet
- Host-Pathogen Interactions in Bacterial MeningitisDocument25 pagesHost-Pathogen Interactions in Bacterial MeningitisEugen TarnovschiNo ratings yet
- 55 61 Brain AbscessDocument7 pages55 61 Brain AbscessNadia OktarinaNo ratings yet
- Intracranial Infection - Prof SunartiniDocument12 pagesIntracranial Infection - Prof SunartiniFranciscus BuwanaNo ratings yet
- Bacterial MeningitisDocument40 pagesBacterial MeningitisDinesh ReddyNo ratings yet
- Meningitis and Acute MeningococcemiaDocument16 pagesMeningitis and Acute Meningococcemiaalfaz lakhaniNo ratings yet
- Bacillus Cereus Bacteremia and Meningoen PDFDocument4 pagesBacillus Cereus Bacteremia and Meningoen PDFdr israrNo ratings yet
- Emerging and Less Common Viral Encephalitides - Chapter 91Document34 pagesEmerging and Less Common Viral Encephalitides - Chapter 91Victro ChongNo ratings yet
- Viral Encephalitis: A Clinician's Guide: ReviewDocument18 pagesViral Encephalitis: A Clinician's Guide: ReviewRandy UlloaNo ratings yet
- What Is Meningitis?Document7 pagesWhat Is Meningitis?laujeroNo ratings yet
- Pubid-848078832 1010 PDFDocument10 pagesPubid-848078832 1010 PDFCocosul Cocosului CocosaruluiNo ratings yet
- Neuroinfections Presentation Diagnosis and Treatment of Meningitis and EncephalitisDocument10 pagesNeuroinfections Presentation Diagnosis and Treatment of Meningitis and EncephalitisZarick SaenzNo ratings yet
- Invasive Cryptococcal Meningitis Presenting As A Skull Base Mass in An Immunocompetent Host: A Case ReportDocument5 pagesInvasive Cryptococcal Meningitis Presenting As A Skull Base Mass in An Immunocompetent Host: A Case ReportAsep RiswandiNo ratings yet
- 2021 - Acute Neurologic Manifestations of Respiratory VirusesDocument17 pages2021 - Acute Neurologic Manifestations of Respiratory VirusesOlga Manco GuzmánNo ratings yet
- Atb Meningitis Coid 2018Document12 pagesAtb Meningitis Coid 2018Danny JacobusNo ratings yet
- MeningitisDocument40 pagesMeningitisHSC UNITEDNo ratings yet
- CNS+Infection+ +Fatima+SeparaDocument20 pagesCNS+Infection+ +Fatima+SeparaMarc Lorenz SeparaNo ratings yet
- Microbiology Assignment. ADocument26 pagesMicrobiology Assignment. ACynthia AbbangNo ratings yet
- CNS Fungal InfectionDocument12 pagesCNS Fungal InfectionMuhammad Yusuf HanifNo ratings yet
- Dental Boards3Document6 pagesDental Boards3Isabelle TanNo ratings yet
- Bio PosterDocument1 pageBio PosterRyan LeeNo ratings yet
- Neuro InfectionsDocument71 pagesNeuro Infectionssrushtideokar0537No ratings yet
- Rna Viruses: EnterovirusDocument4 pagesRna Viruses: EnterovirusYeshaa MiraniNo ratings yet
- تَـلـخـيـص شَـابـتـر ٢٢?Document14 pagesتَـلـخـيـص شَـابـتـر ٢٢?سلطان محمد فوزي سلمانNo ratings yet
- DiphteriaDocument64 pagesDiphteriaOmarNo ratings yet
- Sars-Cov-2 Can Induce Brain and Spine Demyelinating Lesions: Case ReportDocument4 pagesSars-Cov-2 Can Induce Brain and Spine Demyelinating Lesions: Case ReportDino AdijayaNo ratings yet
- Acute Neurological InfectionsDocument6 pagesAcute Neurological Infectionsramon.camara.03No ratings yet
- Meningitis 2Document28 pagesMeningitis 2Mehveen KashifNo ratings yet
- Nervous System InfectionsDocument2 pagesNervous System InfectionsEriQuitaraNo ratings yet
- Bacterial MeningitisDocument26 pagesBacterial MeningitisRai HannaNo ratings yet
- Meningitis: Neonates (65)Document5 pagesMeningitis: Neonates (65)Eugina Naiborhu08No ratings yet
- Mycobacterium Tuberculosis: Bañagado, Noreen B. Bs - Medical Technology 2Document4 pagesMycobacterium Tuberculosis: Bañagado, Noreen B. Bs - Medical Technology 2Noreen Bañagado100% (1)
- Chapter 6 CNS InfectionsDocument5 pagesChapter 6 CNS Infectionssybico.xray.abadclinicNo ratings yet
- Pathogens of The Female Reproductice Site Erika HuertaDocument1 pagePathogens of The Female Reproductice Site Erika HuertaMicroposterNo ratings yet
- Ear Infections - Fatima Khalid - Revised VersionDocument1 pageEar Infections - Fatima Khalid - Revised VersionMicroposterNo ratings yet
- Jackie Tasarz-Liver Pathogens RevisedDocument1 pageJackie Tasarz-Liver Pathogens RevisedMicroposterNo ratings yet
- Infections of The Liver Revised - Nick GriffinDocument1 pageInfections of The Liver Revised - Nick GriffinMicroposterNo ratings yet
- Pathogens of The Vagina-CitationsDocument1 pagePathogens of The Vagina-CitationsMicroposterNo ratings yet
- Pathogens of The Eye Revised - Jenna TuckerDocument1 pagePathogens of The Eye Revised - Jenna TuckerMicroposterNo ratings yet
- Skin Pathogens (Revised) - Courtney ChinnDocument1 pageSkin Pathogens (Revised) - Courtney ChinnMicroposterNo ratings yet
- Pathogens of The Vagina-Annie Espinosa - This Is The Revised VersionDocument1 pagePathogens of The Vagina-Annie Espinosa - This Is The Revised VersionMicroposterNo ratings yet
- Pathogens in The Lungs-Jeffrey DelgadilloDocument1 pagePathogens in The Lungs-Jeffrey DelgadilloMicroposterNo ratings yet
- Savannah Whitington: Your Brain On DrugsDocument1 pageSavannah Whitington: Your Brain On DrugsMicroposterNo ratings yet
- Eye Infections by Allison BakerDocument1 pageEye Infections by Allison BakerMicroposterNo ratings yet
- The Eye - Jenna TuckerDocument1 pageThe Eye - Jenna TuckerjktuckerNo ratings yet
- Liver PathogensDocument1 pageLiver PathogensMicroposterNo ratings yet
- Pathogens of The Female Reproductive Site, Erika HuertaDocument1 pagePathogens of The Female Reproductive Site, Erika HuertaMicroposterNo ratings yet
- Wylie, ClareDocument1 pageWylie, ClareMicroposterNo ratings yet
- Liver PathogensDocument1 pageLiver PathogensMicroposterNo ratings yet
- Pathogens of The Lungs - Michelle CumbaaDocument1 pagePathogens of The Lungs - Michelle CumbaaMicroposterNo ratings yet
- Emily Scroggs Microorganisms Affecting The KidneyDocument1 pageEmily Scroggs Microorganisms Affecting The KidneyMicroposterNo ratings yet
- The Urogenital Tract-Maija SwansonDocument1 pageThe Urogenital Tract-Maija Swansonmswanson5975No ratings yet
- Pathogens of The Gut - Christine ProchnowDocument1 pagePathogens of The Gut - Christine ProchnowMicroposterNo ratings yet
- Pathogens of The Oral Cavity - Karisma ManciasDocument1 pagePathogens of The Oral Cavity - Karisma ManciasMicroposterNo ratings yet
- Pathogens in The Oral CavityDocument1 pagePathogens in The Oral CavityMicroposterNo ratings yet
- Gaby Saenz - The MeningesDocument1 pageGaby Saenz - The MeningesMicroposterNo ratings yet
- EarInfections FatimaKhalidDocument1 pageEarInfections FatimaKhalidMicroposterNo ratings yet
- Gaby Saenz - Works Cited For Meninges PosterDocument2 pagesGaby Saenz - Works Cited For Meninges PosterMicroposterNo ratings yet
- Lung Infections - Jessica de AndaDocument1 pageLung Infections - Jessica de AndaMicroposterNo ratings yet
- Pathogens of The Female Reproductive System - Leah NechamkinDocument1 pagePathogens of The Female Reproductive System - Leah NechamkinMicroposterNo ratings yet
- Pathogens of The Vagina-Annie EspinosaDocument1 pagePathogens of The Vagina-Annie Espinosaannie_espinosa_2No ratings yet
- Class XI Question Paper BIODocument2 pagesClass XI Question Paper BIOShuchi MaheshwariNo ratings yet
- Intro To MicroDocument18 pagesIntro To MicroYasmin Abigail AseriosNo ratings yet
- 2010 UMDNS ThesaurusDocument180 pages2010 UMDNS ThesaurusLee Thoong100% (1)
- Causes of Disease Mark SchemeDocument3 pagesCauses of Disease Mark SchemerkblsistemNo ratings yet
- Social and Preventive Pharmacy Revision Questions CBS PublicationDocument20 pagesSocial and Preventive Pharmacy Revision Questions CBS PublicationAntar GayenNo ratings yet
- Fowl PoxDocument7 pagesFowl PoxLisa VaughnNo ratings yet
- Acquired Immuno Deficiency Syndrome (Aids)Document9 pagesAcquired Immuno Deficiency Syndrome (Aids)alina20No ratings yet
- AdenovirusDocument14 pagesAdenoviruszoomar muzammilNo ratings yet
- Refresher Course On HIV/AIDS (Bob Meyers)Document90 pagesRefresher Course On HIV/AIDS (Bob Meyers)National Press FoundationNo ratings yet
- Animal Experimentation ViewpointsDocument80 pagesAnimal Experimentation ViewpointschristianbarrigaNo ratings yet
- Viral Diseases in Plants-303Document11 pagesViral Diseases in Plants-303Amandeep KaurNo ratings yet
- Animal ScienceDocument19 pagesAnimal ScienceGel Mi AmorNo ratings yet
- Korea Ghsa 2019Document283 pagesKorea Ghsa 2019BRAAAP SQRTNo ratings yet
- Synthesis PaperDocument6 pagesSynthesis Paperapi-252076032No ratings yet
- Public Health - MartiNemerow - Practice Test - P2C1 PDFDocument14 pagesPublic Health - MartiNemerow - Practice Test - P2C1 PDFArly TolentinoNo ratings yet
- The "Oral" History of COVID-19: Primary Infection, Salivary Transmission, and Post-Acute ImplicationsDocument11 pagesThe "Oral" History of COVID-19: Primary Infection, Salivary Transmission, and Post-Acute ImplicationsPramudya NycNo ratings yet
- Lesson 4 - Gene TherapyDocument6 pagesLesson 4 - Gene TherapyKaneki kenNo ratings yet
- Egg Inoculation by C.ngullieDocument59 pagesEgg Inoculation by C.ngullieDR VARSHA A SINGH100% (1)
- Lana Moussa Aleech - Senior Project Research PaperDocument13 pagesLana Moussa Aleech - Senior Project Research Paperapi-655770614No ratings yet
- ToMMVPDIS 10 16 1504 REDocument9 pagesToMMVPDIS 10 16 1504 RETriEka HeryaNo ratings yet
- Louis PasteurDocument2 pagesLouis PasteurMohamed Tayeb SELTNo ratings yet
- Essay: Student Moca Alexandra Year I, Group 12Document35 pagesEssay: Student Moca Alexandra Year I, Group 12Alexutza AlexandraNo ratings yet
- 4-Overview of HIV AIDS - LAC - Dr. Ethyl DanoDocument29 pages4-Overview of HIV AIDS - LAC - Dr. Ethyl DanoNiccolo G. ChiongbianNo ratings yet
- UG Physiology PDFDocument38 pagesUG Physiology PDFAmaradeepika JagannathanNo ratings yet
- Sustainable Cities and Society: Imran Ahmed, Misbah Ahmad, Joel J.P.C. Rodrigues, Gwanggil Jeon, Sadia DinDocument12 pagesSustainable Cities and Society: Imran Ahmed, Misbah Ahmad, Joel J.P.C. Rodrigues, Gwanggil Jeon, Sadia DinHerdiansyah FadlanNo ratings yet
- What Is HIV?Document6 pagesWhat Is HIV?JOSHUA PALMANo ratings yet
- Chronic Autoimmune Mystery Illness Starter Course. Day 1 Video. EditedDocument9 pagesChronic Autoimmune Mystery Illness Starter Course. Day 1 Video. EditedPriyaNo ratings yet
- Water, Water Pollution and Wastewater CharacteristicsDocument26 pagesWater, Water Pollution and Wastewater CharacteristicsAngelo James AmbionNo ratings yet
- VirologyDocument9 pagesVirologyAnisha SarmaNo ratings yet
- Herpes VirusDocument18 pagesHerpes VirusGrinty Babu BabuNo ratings yet