Professional Documents
Culture Documents
Adult Stem Cells For Neuronal Repair
Uploaded by
1alfredo12Original Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Adult Stem Cells For Neuronal Repair
Uploaded by
1alfredo12Copyright:
Available Formats
Stem Cell Research
Adult Stem Cells for Neuronal Repair
Ran Barzilay, Yossef S. Levy PhD, Eldad Melamed MD and Daniel Offen PhD
Laboratory of Neurosciences, Felsenstein Medical Research Center and Department of Neurology, Rabin Medical Center (Beilinson
Campus), Petah Tiqva, Israel
Affiliated to Sackler Faculty of Medicine, Tel Aviv University, Ramat Aviv, Israel
Key words: adult stem cells, neurodegenerative diseases, cell therapy
IMAJ 2006;8:61–66
Stem cell research offers great hope to patients suffering from Cellular therapy for PD: clinical trials
neuronal damage. Stem cell-based regenerative medicine holds Parkinson’s disease affects more than 1% of the population
huge potential to provide a true cure for patients affected by a over age 60 in the western world. PD is characterized by mas-
neurodegenerative disease or who have suffered a stroke. sive loss of specific dopaminergic neurons in the substantia
A stem cell possesses two distinct traits that define it: self- nigra pars compacta and causes severe motor symptoms.
renewal and differentiation capacity. Self-renewal is the process Current treatments for PD comprise mainly dopamine replace-
whereby a single cell gives rise to two daughter cells – one ment and symptom alleviation, rather than a cure for the
being identical to the original cell and the other further com- disease. The type specificity of the damaged cells makes PD
mitted towards a more restricted phenotype. In turn, the further patients ideal candidates for cellular therapy strategies. Cell-
committed progenitor cell, upon specific cues and signals, based therapies for PD have until now focused primarily on
may proceed and differentiate to a mature cell type, a process transplantation of differentiated fetal nigral cells. From the
termed differentiation. beginning of the last decade, different groups have reported
The stem cell population is comprised of two main cell types: reasonably good results in a series of open label trials [1,2].
embryonic stem cells and adult stem cells. The first are derived Benefits were associated with increased striatal fluorodopa up-
from the early blastocyte and are well known for their pluripo- take and evidence of graft-related dopamine release on posi-
tency, the ability to differentiate into virtually any specific cell tron emission tomography. Postmortem studies demonstrated
type in the adult organism. The latter have been known for years long graft survival years after transplantation and a significant
for their capacity to differentiate along their lineage of origin (for re-innervation in the patients’ striata. These results encour-
example, hematopoetic stem cell differentiating into any mature aged the U.S. National Institutes of Health to sponsor two
blood cell type). However, there have been recent reports of the double-blind trials of fetal nigral tissue transplantation. In one
adult stem cell’s ability to differentiate along different lineages study, Freed and colleagues [3] in Colorado implanted solid
than its original organ, showing multipotency and crossing lin- grafts of human embryonic mesencephalic cells derived from
eages in a phenomenon known as “trans-differentiation.” two donors per side into the striata of advanced PD patients.
The exact mechanism responsible for the beneficial effect Despite achieving a modest improvement in motor function
of stem cell cellular treatment has yet to be elucidated. In in patients under age 60, the study failed to meet its primary
principle there are three different approaches. The first is cell endpoint – to improve the quality of life.
replacement; namely, the direct replacement of the degenerated In the second study, Olanow et al. [4] implanted fetal nigral
cells with functional cells, e.g., implantation of differentiated transplants derived from one or four donors per side into the
dopaminergic neurons to replace lost cells in the denervated posterior putamens of PD patients. In this study, graft depos-
nigrostriatal pathway in patients with Parkinson’s disease. The its were placed at closer intervals to try and obtain continu-
second approach is neuroprotection, where transplanted stem ous innervation of the striatum and immunosuppression was
cells provide environmental support to the affected brain cells employed for 6 months. In general, clinical outcome proved to
by secreting essential cytokines and neurotrophic factors, e.g., be better than in the Colorado experiment. Post hoc analysis
intravenous administration of undifferentiated bone marrow noted significant improvement, especially in patients suffering
stromal cells in the treatment of stroke. The third is gene deliv- from milder symptoms prior to transplantation. In addition,
ery, i.e., stem cells serve as a vehicle to deliver specific support- transplanted patients improved as compared to placebo-treated
ive genes in the affected area; e.g., implantation of engineered patients in the first 6 months, but deteriorated afterwards. This
stem cells over-expressing glial-derived neurotrophic factor to deterioration coincided with the cessation of cyclosporine, and
the brains of PD patients will protect the remaining unaffected raised the possibility that immune rejection may have played
neurons and restrain the onset of the disease. a significant role in the long-term outcome of these patients.
However, a substantial percent of patients in both trials suf-
PD = Parkinson’s disease fered dyskinesias that persisted after stopping levodopa (‘off-
• Vol 8 • January 2006 Adult Stem Cells for Neuronal Repair 61
Stem Cell Research
medication’ dyskinesia) that had not been described prior to restorative medicine. Nonetheless, why should one go so far as
transplantation. to induce a blood cell to become a neuron rather than simply
More encouraging results were reported by Mendez and group differentiate the more hymeneal ESC? Well, there are three ma-
in 2002 [5]. In that study, the graft was composed of fetal ventral jor considerations to bear in mind. Firstly, the use of ESCs and
midbrain cell suspension rather than intact tissue as previously fetal tissues raises major ethical issues. Secondly, implanting
described. Patients in these trials did not suffer from dyskinesias ESCs will always involve allograft transplantation, raising immu-
and showed some level of clinical improvement. Isacson et al. nologic consequences while the ultimate use of adult stem cells
[6] recently conducted postmortems of brains of participants in will provide the possibility of autologous cell transplantation.
that study and noted a 3 year survival of cells and a significant Thirdly, adult stem cells, by their very nature of being more
striatal reinnervation. The authors emphasize the importance committed than ESCs, are of less risk than for teratoma forma-
of dopaminergic cell-type specification, stating that the trans- tion [13]. Taking all of this into account, one must consider the
planted cells would optimally acquire the A9 dopaminergic neu- different aspects of adult versus embryonic stem cells for clini-
ron phenotype in order to properly reinnervate the degenerated cal use, the first being more available and less risky, the latter
pathways causing the parkinsonian symptoms. The data obtained more malleable and potent in terms of differentiating capacity.
from the postmortems support the possibility of reconstructing
the degenerated nigrostriatal pathways with functional dopami- Embryonic stem cells to neurons
nergic cells, demonstrating that dopamine neuronal replacement The isolation of human embryonic stem cells has stimulated
cell therapy can be beneficial for PD patients. research aimed at the selective generation of specific cell types
Taken together, these studies highlight the therapeutic poten- for regenerative medicine [14]. In recent years researchers have
tial of cellular therapy for neurodegenerative diseases in general been relentlessly studying the mechanisms involved in the
and PD in particular. However, while the need to establish the embryonic development in rodents and understanding the cues
best transplantation strategy is evident, several questions and signals directing the early inner mass cell towards the neu-
remain unresolved. Choosing the right patients, finding the roectodermal lineage and onward towards maturing into fully
optimal cell source, using immunosuppression and controlling differentiated nerve cells. In principle, stem cell researchers are
the side effects are just some of the issues still waiting to be attempting to apply that knowledge to develop protocols direct-
addressed. Most of all, the controversial use of fetal tissues ing the uncommitted stem cell to become a neuron.
and the necessity to generate a reliable reservoir of dopami- In the PD field of interest, McKay and colleagues [15] re-
nergic neurons has led researchers to explore other stem cell ported the generation of dopaminergic neurons from mouse
resources for possible cell-based therapies. embryonic stem cells in vitro after selecting central nervous
system progenitors from the primary ESC population, expanding
Adult stem cells versus embryonic stem cells the cells in the presence of basic fibroblast growth factor and
Embryonic stem cells represent the classic manifestation of inducing dopaminergic differentiation by withdrawal of the mi-
a stem cell population, showing great capacity of self-renewal togen and addition of cAMP and ascorbic acid. Moreover, their
and differentiation. Massive advancement in embryology and work showed that the addition of molecules previously known
neurogenesis has helped ESCs researchers develop protocols to be involved in the development of midbrain dopaminergic
that generate neural progenitors capable of differentiating into neurons, sonic hedgehog and FGF-8, significantly raised the
neurons, astrocytes and oligodendrocytes from human ESCs [7]. expression of tyrosine hydroxylase, the rate-limiting enzyme in
On the other hand, there are the adult stem cells, which are dopamine synthesis. The induced cells expressed specific mark-
comprised of several subtypes of cell populations. The most stud- ers of dopamine neurons, secreted dopamine in response to
ied adult stem cell population is the hematopoetic, which reside depolarization and showed electrophysiologic properties similar
in the bone marrow and have long been known for their ability to neurons. Since then several groups have been able to gener-
to replenish the blood system after immunologic irradiation of ate dopamine-producing cells from human ESCs by following or
patients suffering from hematologic malignancies, by differentiat- optimizing the differentiation protocol, achieving a higher yield
ing along the blood system hierarchy. However, in recent years of differentiated dopaminergic cells [16].
a large body of evidence indicated that some subpopulations of When transplantations of ESC-derived dopaminergic neurons
adult stem cells are capable of differentiating into mature cells were performed in animal models of PD, success was only par-
different from their original lineage, a phenomenon termed trans- tial. Takagi et al. [17] managed to generate dopaminergic neu-
differentiation. These multipotent adult stem cells reside in vari- rons from primate ESCs and achieved behavioral improvement
ous compartments in the mature organism and display plasticity upon transplantation into MPTP (1-methyl-4-phenyl-1,2,3,6-tet-
initially thought to belong exclusively to ESCs. Those cells have rahydropyridine) models of primates. Nevertheless, histologic
been isolated from brain [8], bone marrow [9], skin [10], fat [11], analysis revealed a low rate of TH-positive surviving neurons
skeletal muscle [12] and other visceral organs. after transplantations.
These findings widened the spectrum of possible sources for
FGF = fibroblast growth factor
ESC = embryonic stem cell TH = tyrosine hydroxylase
62 R. Barzilay et al. • Vol 8 • January 2006
Stem Cell Research
Xu et al. [18] reported a symptomatic improvement in a On the one hand, NSCs are a long way ahead of ESCs in their
rat 6-hydroxydopamine model of PD after transplantation of commitment to the mature neuronal or glial phenotype and are
mouse ESC-derived neural progenitors, again despite a low less prone than ESCs to form teratomas. On the other hand,
survival rate of cells 6 weeks after transplantation (2%). As they do not have to cross lineage borders in order to differ-
for human ESCs, Reubinoff and co-workers [19] are the only entiate to mature CNS cells in comparison to adult stem cells
group to date to report a behavioral improvement in a PD rat residing in other compartments.
model after transplantation of hESC-derived neural progenitors. In rodents, Gritty and team [24] reported the expansion of
The authors point to a correlation between TH+ cell presence NSCs from the olfactory bulb of adult mice following bulbec-
and the degree of behavioral improvement. But once again, tomy. NSCs proliferated extensively in the presence of EGF
the TH+ population was significantly reduced as compared to and b-FGF before being induced to differentiate into neurons,
studies conducted in vitro, suggesting the need to direct cells to astrocytes and oligodentrocytes upon exposure to different
dopaminergic differentiation prior to transplantation. However, growth factors and cytokines. Moe and colleagues [25] recently
researchers who tried implanting ESC-derived dopaminergic described the efficient formation of a functional neural network
neurons to animal models have not yet achieved a significant originated from a single cell of the adult human brain. NSCs
improvement in PD models [20,21]. Possible explanations for were obtained from biopsies and expanded at the single cell
these disappointing results could be the low survival rate of level to form a large cell population. Upon withdrawal of mi-
TH+ neurons due to host immune response and apoptotic be- togens and the addition of fetal calf serum, cells matured and
havior of dopaminergic cells in the host brain, perhaps due to expressed glutamate receptors, vesicular glutamate transporter
limited success of the differentiation protocol. Moreover, some and other mature neuronal proteins such as NeuN. Moreover,
of these studies have also reported ectopic non-neural protein patch clamp analysis showed that the cells possess mature
expression of the transplanted cells in the brain, suggesting the electrophysiologic properties. The authors argue that NSCs can
possibility of teratoma formation [20,21]. easily be harvested following neuroendoscopy from the ventricu-
lar wall and may serve as a resource for autologous transplan-
Adult stem cells to neurons tation in the future.
The generation of differentiated cells with a mature neuronal
or astroglial phenotype has been achieved using cells from the Bone marrow-derived stem cells
adult brain, bone marrow, skin, fat, muscle and more. It would The therapeutic potential of bone marrow transplantation has
be fair to say that a consensus is emerging among scientists to- already proven itself in the treatment of hematologic malignan-
day that there are stem cells in every compartment of the adult cies. Whole bone marrow contains two distinct cell popula-
body, although the role of some of them in adult life is still tions, the hematopoetic cells and the non-hematopoetic cells.
unknown. The advantages of autologous stem cell transplanta- Hematopoetic stem cells are well characterized as CD34+ cells
tion are a great incentive for researchers to develop protocols and have been used in the clinic with major success for hema-
that will eventually allow medicine to utilize adult stem cell for tologic disorders. The non-hematopoetic cells are widely known
neuronal repair [Figure 1]. for their essential role in nourishing the HSCs by secreting
different growth factors and have also been used as a feeder
Brain-derived neural stem cells layer supporting the development of ESCs. In the 1970s, Frie-
It was long considered an axiom that the brain is deprived of denstein et al. [26] found a stem cell population negative for
the ability to produce new neurons. However, Gage [8] pre- HSC markers. Those cells showed the capacity to self-renew and
sented evidence for the existence of adult neural stem cells in differentiate along the mesenchymal lineage into bone, fat and
the adult brain that are able to differentiate into all three neu- cartilage cells. These cells are the bone marrow stromal cells,
ral cell types: neurons, astocytes, oligodentrocytes. Those stem also referred to as mesenchymal stem cells. Recent studies
cells are characterized by their ability to form neurospheres, have shown that under specific conditions, MSCs are capable
floating cell aggregates widely expressing the filament nestin, of differentiating to other mesodermal lineages, extending their
and their ability to expand extensively in the presence of the plastic potential.
mitogens, epithelial growth factor and b-FGF. The subventricular
zone and the dendate gyrus are known for their relatively abun- Mesenchymal stem cells
dant population of stem cells, but recent work suggests that Woodbury et al. [27] were the first to report the MSC capacity
even the striatum and substantia nigra contain a subpopulation to break the mesenchymal lineage barrier and to differentiate
of quiescent stem cells [22,23]. Neural stem cells have been of into neurons. Rat and human MSCs were expanded before be-
great interest to scientists seeking a cure for neuronal damage. ing induced to differentiate in a serum-free medium containing
β-mercaptoethanol and other antioxidants. Upon exposure to
induction medium, MSCs dramatically changed their morphol-
NSCs = neural stem cells
CNS = central nervous system ogy from long flat spindle-shaped cells to contracted rounded
EGF = epithelial growth factor cells with long extensions resembling neurite outgrowths. The
HSCs = hematopoietic stem cells induced cells expressed typical neuronal proteins, such as
• Vol 8 • January 2006 Adult Stem Cells for Neuronal Repair 63
Stem Cell Research
to rats transplanted with undifferentiated MSCs. The authors
concluded that not only are MSCs capable of nerve regeneration
but that differentiation in vitro prior to transplantation is essen-
tial to achieve optimal results.
The generation of dopaminergic neurons is of utmost im-
portance in the quest to find a cure for PD. Storch et al. [31]
described the generation of neural stem cells from human
MSCs. These cells expanded in floating aggregates resembling
neurospheres, displayed clonal capacity and were differentiated
at the single cell level to both neurons and glial cells. More-
over, after induction with brain-derived neurotrophic factor, cells
secreted dopamine in response to depolarization and showed
electrophysiologic properties typical of dopaminergic neurons.
In our laboratory, Levy and co-workers [32] detailed the
induction of the neuronal specific enolase promoter and
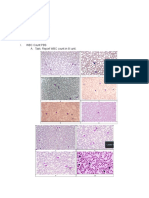
Figure 1. Schematic illustration of autologous bone marrow- the induced expression of other neuronal markers following
derived mesenchymal stem cell transplantation for cellular neuronal differentiation of mouse MSCs. Moreover, when
replacement or neuroprotection. dopaminergic induced mouse MSCs were transplanted into
a mouse 6-hydroxydopamine model of PD, we observed a
neuron-specific enolase, neurofilament M and the neuronal reduction in rotational behavior as compared to mice injected
nuclear-specific antigen NeuN. Sanchez-Ramos and collabora- with saline [paper in preparation]. As for human MSCs, we
tors [28] reported the differentiation of human and rat MSCs recently reported the efficient dopaminergic induction of human
to both neuronal and glial cells in the presence of retinoic acid MSCs [presented at the Third ISSCR Conference]. MSCs were
and brain-derived neurotrophic factor. Moreover, their experi- expanded and characterized for the typical MSC cell surface
ments showed that co-culture of MSCs with NSCs derived from phenotype; the cells also ubiquitously expressed the typical
neonate mice or human fetal tissues promoted the neuronal neuronal progenitor marker nestin. Upon exposure to various
differentiation of MSCs. differentiation media the induced cells acquired a typical neural
To determine whether trans-differentiation of MSCs to neu- morphology, expressed a variety of neuronal proteins including
rons could also occur in vivo, Munoz-Elias et al. [29] transplanted TH, and secreted dopamine in response to depolarization
rat MSCs into the rat embryo. The transplanted cells integrated [Figure 2].
in the brain parenchyma and followed specific developmental The mechanism whereby clinical improvement following
cues and migratory pathways of the rat neurogenesis. Abundant MSCs transplantation is achieved has yet to be elucidated. One
donor cells were detected in the neocortex, hippocampi, cerebel- possibility is that the graft cells functionally replace the dam-
lar peduncles, colliculi, thalamus, midbrain,
forebrain germinal zones, rostral migra-
tory stream and olfactory bulbs. The cells
expressed different gene products and mor-
phologies in a region-specific manner, appar-
ently exhibiting the plastic ability to respond
specifically to different microenvironments.
Dezawa et al. [30] reported on the sciatic
nerve regeneration induced by transplanta-
tion of schwann cells derived from MSCs.
Rat MSCs were expanded and induced in
vitro to express a schwann cell phenotype
expressing S-100, O4 and GFAP follow-
ing incubation with b-FGF, platelet-derived
growth factor, forskolin and retinoic acid.
The induced cells were transplanted into the
proximal site of the dissected sciatic nerve. Figure 2. Neural induced human mesenchymal stem cells display neural
Within 3 weeks of the operation, trans- morphology and express neuronal proteins. [A] Untreated human mesenchymal
stem cells. [B] Mesenchymal stem cells following neuronal induction. [C]
planted cells elicited vigorous regeneration, Mesenchymal stem cells following neuronal induction stained for Tuj1 (β-tubulinIII).
accompanied by remyelinization, compared [D] Mesenchymal stem cell following neuronal induction demonstrates astrocyte-like
morphology. [E] Mesenchymal stem cells following neuronal induction stained for
MSCs = mesenchymal stem cells tyrosine hydroxylase.
64 R. Barzilay et al. • Vol 8 • January 2006
Stem Cell Research
aged cell population following trans-differentiation in vivo or in derived mesenchymal stem cells were isolated and propagated
vitro prior to transplantation. However, some researchers claim in the presence of EGF and b-FGF and expressed both neural
that trans-differentiation is at most a marginal phenomenon stem cell transcripts and proteins. The skin-derived neural pro-
that cannot account for the observed clinical improvement. genitors were then exposed to rodent astrocytes-conditioned
Chopp and colleagues [33–35] administered human MSCs in- medium. Upon induction the cells acquired typical neuronal
travenously into animal models of various neurologic disorders. morphology, expressed neuronal and glial proteins, and ex-
These included the MPTP mouse model of PD, a stroke model hibited a reversible increase in intracellular calcium in direct
in rats, and recently in an experimental allergic encephalitis response to potassium-mediated depolarization, which is con-
model in rats bearing symptoms similar to multiple sclerosis. sistent with the presence of voltage-gated calcium channels.
In all three experiments a significant clinical improvement was In another paper published in the Lancet in the same year,
observed. Intriguingly, immunohistochemistry revealed the abun- Alessandri and co-workers [12] described the neural induction
dant presence of administered MSCs near the damaged areas in of skeletal muscle-derived stem cell. Stem cells were obtained
the brain in comparison to unlesioned controls, suggesting that from brachioradialis muscle samples after tissue was minced
MSCs follow chemotactic cues and migrate through inflamma- and trypsinized. Cells were cultured in a typical neural stem cell
tory pathways. However, Chopp and team attribute the clinical culture medium containing EGF and b-FGF. Following massive
improvement to the supporting effect of the MSCs rather than cell death, the surviving cells proliferated and clonal analysis
to cell replacement following trans-differentiation. These investi- confirmed their stem cell qualities. Experiments in vitro indi-
gators claim that although some cells stain positive to neuronal cated that the muscle-derived stem cells could be induced to
or astroglial markers, their level of expression is not sufficient express both neuronal and glial markers. However, an attempt
to account for the clinical improvement. The therapeutic effect to observe differentiation in vivo did not succeed because mus-
is rather related to a secretion of neurotrophic factors such as cle-derived stem cells transplanted into an injured rat’s spinal
brain-derived neurotrophic factor, nerve growth factor and other cord were found negative for neural specific marker staining.
factors such as vascular endothelial growth factor and inflam-
matory modulatory molecules. Conclusions and future prospects
Stem cell therapy offers great hope for patients suffering from
Cord blood stem cells disease for which there is currently no cure. The notion of a
Umbilical cord stem cells are considered an important source scientist able to generate true cellular replacements to substi-
for autologous cellular therapies. Like bone marrow, cord blood tute defective cells in humans has sparked the imagination of
consists of both a hematopoietic and a non-hematopoietic stem doctors and researchers in all disciplines of modern medicine.
cell population. In recent years UCBSC transplantations have Cellular treatment in cancer patients using hematopoietic stem
proved effective for treating children with hematologic diseases. cells has been used efficiently for more than 50 years and is
However, the relatively small amount of cells harvested from a now practiced routinely in the clinic. Stem cell transplantation
single cord makes it crucial to further develop new technologies in parkinsonian patients has proved feasible in several clinical
that enable sufficient expansion for the treatment of adult pa- trials.
tients. A review recently published by Sanberg et al. [36] sheds In order to harness the full potential of stem cell therapy,
light on the possible potential of UCBSCs for neuronal repair. science must overcome moral, logistic and clinical challenges.
Like its counterpart stem cell population in the bone marrow, Adult stem cells appear to be the ideal candidates to serve as
UCBSCs have shown plasticity in vitro by differentiating into the cellular reservoir. In comparison to embryonic stem cells,
both neurons and glia cells. Reports on the therapeutic effect adult stem cell harvesting does not involve the controversial
of UCBSCs in vivo are still limited, but a couple of papers noted use of embryos or eggs. They will optimally be transplanted
the beneficial effect of intravenous UCBSC transplantation in an autologously, decreasing to a minimum the risk of immune
animal model of stroke and an animal model of amyotrophic rejection and, because of their more restricted differentiation
lateral sclerosis. However, the mechanism of the clinical im- potential, are considered less prone to form tumors in the host.
provement was not elucidated as there was no conclusive evi- Patients afflicted with neuronal damage, neurodegenerative
dence of trans-differentiation, suggesting a more neuroprotective disorders, spinal cord injury or stroke suffer the consequences
effect by inducing growth factor secretion or neovascularization. of insufficient neurogenesis and cell renewal in their affected
nervous system. Current therapies are mostly symptomatic and
Other adult stem cells for neuronal repair in some cases inefficient. The tireless efforts being invested in
A number of groups worldwide have observed trans-differen- developing new technologies in stem cell research will hopefully
tiation to neural cells in stem cells derived from tissues other yield the knowledge that will enable doctors to present patients
than brain, bone marrow or cord blood. In a report published with a cure.
in the Lancet in 2004, Joanides et al. [10] described the efficient
generation of neural precursors from adult human skin. Dermis- Acknowledgments. This work was performed in partial fulfill-
ment of the requirements for a PhD degree of Ran Barzilay, Sack-
UCBSCs = umbilical cord stem cells ler Faculty of Medicine, Tel Aviv University, Israel. This work was
• Vol 8 • January 2006 Adult Stem Cells for Neuronal Repair 65
Stem Cell Research
supported, in part, by the Israel Ministry of Health and the Nation- 19. Ben-Hur T, Idelson M, Reubinoff BE, et al. Transplantation of hu-
al Parkinson Foundation, Miami, FL, USA, and the Norma and Alan man embryonic stem cell-derived neural progenitors improves be-
Aufzein Chair for Research in Parkinson’s Disease, Tel Aviv University, havioral deficit in Parkinsonian rats. Stem Cells 2004;22:1246–55.
Israel. The authors thank Alex Burshtein and Shira Goldstein, Labo- 20. Park CH, Minn YK, Lee JY, et al. In vitro and in vivo analyses of
human embryonic stem cell-derived dopamine neurons. J Neuro-
ratory of Neurosciences, Felsenstein Medical Research Center, for
chem 2005;92:1265–76.
their assistance and to Moti Barzilay for the preparation of Figure 1. 21. Zeng X, Cai J, Freed WJ, et al. Dopaminergic differentiation of
human embryonic stem cells. Stem Cells 2004;22:925–40.
22. Tate MC, Garcia AJ, Keselowsky BG, et al. Specific beta1 integ-
References rins mediate adhesion, migration, and differentiation of neural
1. Freed CR, Breeze RE, Rosenberg NL, et al. Survival of implant- progenitors derived from the embryonic striatum. Mol Cell Neurosci
ed fetal dopamine cells and neurologic improvement 12 to 46 2004;27:22–31.
months after transplantation for Parkinson’s disease. N Engl J 23. Zhao M, Momma S, Delfani K, et al. Evidence for neurogenesis
Med 1992;327:1549–55. in the adult mammalian substantia nigra. Proc Natl Acad Sci USA
2. Lindvall O, Brundin P, Widner H, et al. Grafts of fetal dopamine 2003;100:7925–30.
neurons survive and improve motor function in Parkinson’s dis- 24. Gritti A, Bonfanti L, Doetsch F, et al. Multipotent neural stem
ease. Science 1990;247:574–7. cells reside into the rostral extension and olfactory bulb of adult
3. Freed CR, Greene PE, Breeze RE, et al. Transplantation of embry- rodents. J Neurosci 2002;22:437–45.
onic dopamine neurons for severe Parkinson’s disease. N Engl J 25. Moe MC, Westerlund U, Varghese M, Berg-Johnsen J, Svensson
Med 2001;344:710–19. M, Langmoen IA. Development of neuronal networks from single
4. Olanow CW, Goetz CG, Kordower JH, et al. A double-blind con- stem cells harvested from the adult human brain. Neurosurgery
trolled trial of bilateral fetal nigral transplantation in Parkinson’s 2005;56:1182–8.
disease. Ann Neurol 2003;54:403–14. 26. Friedenstein AJ, Gorskaja JF, Kulagina NN. Fibroblast precursors
5. Mendez I, Dagher A, Hong M, et al. Simultaneous intrastria- in normal and irradiated mouse hematopoietic organs. Exp Hema-
tal and intranigral fetal dopaminergic grafts in patients with tol 1976;4:267–74.
Parkinson disease: a pilot study. Report of 3 cases. J Neurosurg 27. Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and hu-
2002;96:589–96. man bone marrow stromal cells differentiate into neurons. J Neu-
6. Mendez I, Sanchez-Pernaute R, Isacson O, et al. Cell type analy- rosci Res 2000;61:364–70.
sis of functional fetal dopamine cell suspension transplants in 28. Sanchez-Ramos J, Song S, Sanberg PR, et al. Adult bone marrow
the striatum and substantia nigra of patients with Parkinson’s stromal cells differentiate into neural cells in vitro. Exp Neurol
disease. Brain 2005;128:1498–510. Epub 2005 May 4. 2000;164:247–56.
7. Reubinoff BE, Itsykson P, Ben-Hur T, et al. Neural progenitors 29. Munoz-Elias G, Marcus AJ, Coyne TM, Woodbury D, Black IB.
from human embryonic stem cells. Nat Biotechnol 2001;19:1134–40. Adult bone marrow stromal cells in the embryonic brain: engraft-
8. Gage FH. Mammalian neural stem cells [Review]. Science 2000; ment, migration, differentiation, and long-term survival. J Neurosci
287:1433–8. 2004;24:4585–95.
9. Prockop DJ. Marrow stromal cells as stem cells for nonhemato- 30. Dezawa M, Takahashi I, Esaki M, Takano M, Sawada H. Sci-
poietic tissues [Review]. Science 1997;276:71–4. atic nerve regeneration in rats induced by transplantation of
10. Joannides A, Gaughwin P, Schwiening C, Chandran S. Efficient in vitro differentiated bone-marrow stromal cells. Eur J Neurosci
generation of neural precursors from adult human skin: astro- 2001;14:1771–6.
cytes promote neurogenesis from skin-derived stem cells. Lancet 31. Hermann A, Gastl R, Storch A, et al. Efficient generation of neu-
2004;364:172–8. ral stem cell-like cells from adult human bone marrow stromal
11. Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a cells. J Cell Sci 2004;117:4411–22.
source of multipotent stem cells. Mol Biol Cell 2002;13:4279–95. 32. Levy YS, Merims D, Panet H, Barhum Y, Melamed E, Offen D.
12. Alessandri G, Pagano S, Bez A, et al. Isolation and culture of Induction of neuron-specific enolase promoter and neuronal
human muscle-derived stem cells able to differentiate into myo- markers in differentiated mouse bone marrow stromal cells. J Mol
genic and neurogenic cell lineages. Lancet 2004;364:1872–83. Neurosci 2003;21:121–32.
13. Fujikawa T, Oh SH, Pi L, Hatch HM, Shupe T, Petersen BE. Tera- 33. Li Y, Chen J, Wang L, Zhang L, Lu M, Chopp M. Intracerebral
toma formation leads to failure of treatment for type I diabetes transplantation of bone marrow stromal cells in a 1-methyl-4-
using embryonic stem cell-derived insulin-producing cells. Am J phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s dis-
Pathol 2005;166:1781–91. ease. Neurosci Lett 2001;316:67–70.
14. Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem 34. Li Y, Chen J, Chopp M, et al. Human marrow stromal cell ther-
cell lines derived from human blastocysts. Science 1998;282:1145–7. apy for stroke in rat: neurotrophins and functional recovery.
Erratum 1998;282:1827. Neurology 2002;59:514–23.
15. Lee SH, Lumelsky N, Studer L, Auerbach JM, McKay RD. Efficient 35. Zhang J, Li Y, Chopp M, et al. Human bone marrow stromal cell
generation of midbrain and hindbrain neurons from mouse em- treatment improves neurological functional recovery in EAE mice.
bryonic stem cells. Nat Biotechnol 2000;18:675–9. Exp Neurol 2005;195:16–26.
16. Perrier AL, Tabar V, Barberi T, et al. Derivation of midbrain dopa- 36. Sanberg PR, Willing AE, Garbuzova-Davis S, et al. Umbilical cord
mine neurons from human embryonic stem cells. Proc Natl Acad blood-derived stem cells and brain repair. Ann N Y Acad Sci
Sci USA 2004;101:12543–8. 2005;1049:67–83.
17. Takagi Y, Takahashi J, Saiki H, et al. Dopaminergic neurons gen-
erated from monkey embryonic stem cells function in a Parkin-
son primate model. J Clin Invest 2005;115:102–9. Correspondence: Dr. D. Offen, Felsenstein Medical Research Cen-
18. Xu H, Fan X, Wu X, Tang J, Yang H. Neural precursor cells dif- ter, Beilinson Campus, Petah Tiqwa 49100, Israel.
ferentiated from mouse embryonic stem cells relieve symptomatic Phone: (972-3) 937-6130
motor behavior in a rat model of Parkinson’s disease. Biochem Fax: (972-3) 921-1478
Biophys Res Commun 2005;326:115–22. email: doffen@post.tau.ac.il
66 R. Barzilay et al. • Vol 8 • January 2006
You might also like
- Stem Cells in Regenerative Medicine: Carpe Diem – Carpe Vitam!From EverandStem Cells in Regenerative Medicine: Carpe Diem – Carpe Vitam!Rating: 2.5 out of 5 stars2.5/5 (3)
- Research Papers On Stem Cell TherapyDocument6 pagesResearch Papers On Stem Cell Therapyafeatoxmo100% (1)
- The Concept of Radiation-Enhanced Stem Cell Differentiation: Research ArticleDocument8 pagesThe Concept of Radiation-Enhanced Stem Cell Differentiation: Research ArticledwinugrohojuandaNo ratings yet
- Alzheimers Research E-Journal Issue 10 2012Document16 pagesAlzheimers Research E-Journal Issue 10 2012niallw1974No ratings yet
- Research Paper On Stem Cell TherapyDocument6 pagesResearch Paper On Stem Cell Therapyhfuwwbvkg100% (1)
- Stem Cell 1Document11 pagesStem Cell 1توفيق باعبادNo ratings yet
- LESSON 10 Mod3Document8 pagesLESSON 10 Mod3jasper pachingelNo ratings yet
- Defining The Pathways of Heart RegenerationDocument2 pagesDefining The Pathways of Heart RegenerationIoannis Chaidos TransitusNo ratings yet
- NRR-17-1867.primataDocument9 pagesNRR-17-1867.primatawi bowoNo ratings yet
- Stem Cells DissertationDocument6 pagesStem Cells DissertationHelpWithCollegePaperWritingUK100% (1)
- Cell Based Therapeutic Strategies For Treatment Of.6Document10 pagesCell Based Therapeutic Strategies For Treatment Of.6Tamara MuñozNo ratings yet
- Prof. Datuk DR A Rahman - Stem Cells - The Current State of Its Science and EthicsDocument34 pagesProf. Datuk DR A Rahman - Stem Cells - The Current State of Its Science and EthicsKPJConferenceNo ratings yet
- Long-Term Clinical Outcome of Fetal Cell Transplantation For Parkinson Disease Two Case ReportsDocument5 pagesLong-Term Clinical Outcome of Fetal Cell Transplantation For Parkinson Disease Two Case Reportsjust for download matterNo ratings yet
- Cranial Manipulation Affects Cholinergic Pathway Gene Expression in Aged RatsDocument9 pagesCranial Manipulation Affects Cholinergic Pathway Gene Expression in Aged RatsTameemNo ratings yet
- Parkinsons TreatmeantDocument7 pagesParkinsons Treatmeantkhadija khokhawalaNo ratings yet
- Cardiac Stem Cell Therapy: An Overview: AKM M Islam, AAS Majumder, F Doza, MM Rahman, H JesminDocument15 pagesCardiac Stem Cell Therapy: An Overview: AKM M Islam, AAS Majumder, F Doza, MM Rahman, H JesminNavojit ChowdhuryNo ratings yet
- Treating Parkinson Disease With Adult Stem CellsDocument13 pagesTreating Parkinson Disease With Adult Stem Cellssyahrul ridengNo ratings yet
- Stem Cell-Based Therapy For Neurodegenerative DiseasesDocument144 pagesStem Cell-Based Therapy For Neurodegenerative Diseaseshelikobakter.No ratings yet
- Baker Petersen 2018 Cellular Senescence in Brain Aging and Neurodegenerative Diseases - RAFAELA FAUSTINO LACERDA de SOUZADocument10 pagesBaker Petersen 2018 Cellular Senescence in Brain Aging and Neurodegenerative Diseases - RAFAELA FAUSTINO LACERDA de SOUZAraquel rodriguesNo ratings yet
- Role Stem Cells Health Science Medicine 2638 8235 Pvpe 19 104Document4 pagesRole Stem Cells Health Science Medicine 2638 8235 Pvpe 19 104PromiseNo ratings yet
- FC501 Essay 2Document5 pagesFC501 Essay 2valNo ratings yet
- STS - Stem Cell TherapyDocument29 pagesSTS - Stem Cell TherapyMec AmilasanNo ratings yet
- Stem Cells Thesis PDFDocument4 pagesStem Cells Thesis PDFHelpWithWritingPapersPittsburgh100% (2)
- ParkinsonDocument4 pagesParkinsonbb pigNo ratings yet
- Stem CellsDocument58 pagesStem CellsRakesh Kumar100% (2)
- Stem Cell Therapy For Cerebral Palsy: ReviewDocument9 pagesStem Cell Therapy For Cerebral Palsy: Reviewallyssa rahmadittaNo ratings yet
- Bedeschi 2006Document8 pagesBedeschi 2006Modou NianeNo ratings yet
- Chapter 8Document12 pagesChapter 8lala_0No ratings yet
- Insights New111Document13 pagesInsights New111cassidy conchaNo ratings yet
- Assingement#2 Stem CellDocument6 pagesAssingement#2 Stem Cellhaseeb ShafaatNo ratings yet
- Progress in The ReprogrammingDocument13 pagesProgress in The ReprogrammingnembutalNo ratings yet
- Ajpcell 00166 2015Document22 pagesAjpcell 00166 2015JasonNo ratings yet
- Farin 2009Document25 pagesFarin 2009Laura Duarte RojasNo ratings yet
- Stem Cell Research News PaperDocument5 pagesStem Cell Research News Paperafnkylqbkfazpp100% (1)
- Induce Pluripotent Stem Cell Methods, Development and Advancesn 02 FebDocument11 pagesInduce Pluripotent Stem Cell Methods, Development and Advancesn 02 FebGJESRNo ratings yet
- Effects of Neural Stem Cell TransplantationDocument11 pagesEffects of Neural Stem Cell TransplantationChouaib OujhainNo ratings yet
- Stem Cell Research Paper AbstractDocument4 pagesStem Cell Research Paper Abstractzejasyvkg100% (1)
- Fnins 14 558532Document16 pagesFnins 14 558532anita putri effendiNo ratings yet
- Central Nervous System Juvenile Xantogranuloma A CDocument96 pagesCentral Nervous System Juvenile Xantogranuloma A CAndrés WunderwaldNo ratings yet
- Prentice Testimony HCBAR GO Fetal Tissue 12.13.18Document10 pagesPrentice Testimony HCBAR GO Fetal Tissue 12.13.18Umang PrajapatiNo ratings yet
- OPERA OcrelizumabDocument14 pagesOPERA OcrelizumabalmarazneurologiaNo ratings yet
- Script WPS OfficeDocument2 pagesScript WPS OfficeIvy VergaraNo ratings yet
- 352 FullDocument3 pages352 FullAlexandria Firdaus Al-farisyNo ratings yet
- A Randomized, Placebo-Controlled Trial of Natalizumab For Relapsing Multiple SclerosisDocument12 pagesA Randomized, Placebo-Controlled Trial of Natalizumab For Relapsing Multiple Sclerosisarnoldo VazquezNo ratings yet
- Lab AbstractsDocument1,576 pagesLab AbstractsMichael DaleyNo ratings yet
- Stem Cell Therapy (Final Paper)Document18 pagesStem Cell Therapy (Final Paper)pax romanaNo ratings yet
- Term Paper On Stem Cell TherapyDocument5 pagesTerm Paper On Stem Cell Therapyc5pbp8dk100% (1)
- A New Approach To Cerebral Palsy Treatment DiscussDocument13 pagesA New Approach To Cerebral Palsy Treatment DiscussArun KumarNo ratings yet
- Srinivasan 2016Document16 pagesSrinivasan 2016Zeljko TomljanovicNo ratings yet
- 17 ManuscriptDocument10 pages17 ManuscriptBaru Chandrasekhar RaoNo ratings yet
- Gene Delivery DissertationDocument7 pagesGene Delivery DissertationPayToWritePaperSingapore100% (1)
- (Chandrakanthan Et Al., 2016) PDGF-AB and 5-Azacytidine Induce Conversion of Somatic Cells Into Tissue-Regenerative Multipotent Stem CellsDocument10 pages(Chandrakanthan Et Al., 2016) PDGF-AB and 5-Azacytidine Induce Conversion of Somatic Cells Into Tissue-Regenerative Multipotent Stem CellsJulio dR AltavasNo ratings yet
- Group 1 Reflection: Members: Boh Guias Mangubat Oñate Tayko VerdonDocument3 pagesGroup 1 Reflection: Members: Boh Guias Mangubat Oñate Tayko VerdonTonette Hatamosa VerdonNo ratings yet
- Achievements in Stem Cell and Regenerative MedicineDocument35 pagesAchievements in Stem Cell and Regenerative MedicineKajelcha FikaduNo ratings yet
- Stem Cells and Cancer Stem Cells, Volume 2 - Stem Cells and Cancer Stem Cells, Therapeutic Applications in Disease and Injury - Volume 2Document409 pagesStem Cells and Cancer Stem Cells, Volume 2 - Stem Cells and Cancer Stem Cells, Therapeutic Applications in Disease and Injury - Volume 2ArtanNo ratings yet
- Advantages DismadDocument1 pageAdvantages DismadDaniela Garcia VasquezNo ratings yet
- The Disruption of Profiling of Serotonergic Neurons, Results in Autism-Related BehaviorsDocument22 pagesThe Disruption of Profiling of Serotonergic Neurons, Results in Autism-Related BehaviorsAngelinni Taglioni StangeNo ratings yet
- 0004 282X Anp 72 06 451Document6 pages0004 282X Anp 72 06 451Okhuarobo SamuelNo ratings yet
- 2021 04 8 RegMed CV Final3Document59 pages2021 04 8 RegMed CV Final3Obed IrwantoNo ratings yet
- Aging ImuneDocument9 pagesAging ImuneSNo ratings yet
- Odio Argentina, Su Gente Y Gobierno!!!!!!!!!Document19 pagesOdio Argentina, Su Gente Y Gobierno!!!!!!!!!1alfredo12No ratings yet
- Odio Argentina, Su Gente Y Gobierno!!!!!!!!!Document19 pagesOdio Argentina, Su Gente Y Gobierno!!!!!!!!!1alfredo12No ratings yet
- Chart One: For People Born Before May 24, 1934: U.S. CitizenDocument5 pagesChart One: For People Born Before May 24, 1934: U.S. Citizen1alfredo12No ratings yet
- Forging New Ties-FINALDocument20 pagesForging New Ties-FINAL1alfredo12No ratings yet
- WRR 114Document8 pagesWRR 1141alfredo12No ratings yet
- Rat Adult Stem Cells (Marrow Stromal Cells) Engraft and Differentiate in Chick Embryos Without Evidence of Cell FusionDocument4 pagesRat Adult Stem Cells (Marrow Stromal Cells) Engraft and Differentiate in Chick Embryos Without Evidence of Cell Fusion1alfredo12100% (1)
- Ria LeptinDocument37 pagesRia Leptin1alfredo12100% (1)
- CH 01Document12 pagesCH 01TahmidNo ratings yet
- Macrophages: Development and Tissue Specialization: Chen Varol, Alexander Mildner, and Steffen JungDocument35 pagesMacrophages: Development and Tissue Specialization: Chen Varol, Alexander Mildner, and Steffen JungGustavo VilcapazaNo ratings yet
- Chromatin Accessibility Dynamics During Chemical Induction of PL 20181Document20 pagesChromatin Accessibility Dynamics During Chemical Induction of PL 20181Benn BasilNo ratings yet
- (Hema) 1.1 Intro To Hema (Perez) - PANDADocument6 pages(Hema) 1.1 Intro To Hema (Perez) - PANDATony DawaNo ratings yet
- T Cell Development Thymic Education of T CellDocument34 pagesT Cell Development Thymic Education of T Cellapi-273068056No ratings yet
- A Guide To Human Immune Cell Characterization by Flow CytometryDocument5 pagesA Guide To Human Immune Cell Characterization by Flow CytometryLeo TolstoyNo ratings yet
- HEMATOLOGY 1 Hematopoiesis Notes 1Document10 pagesHEMATOLOGY 1 Hematopoiesis Notes 1sansastarkNo ratings yet
- Hematology 425, LeukopoiesisDocument56 pagesHematology 425, LeukopoiesishanzukikNo ratings yet
- Lecture 3 GametogenesisDocument28 pagesLecture 3 GametogenesisAparna PSNo ratings yet
- Dr. Artrien Adhi Putri, SP.P (K), M.biomedDocument44 pagesDr. Artrien Adhi Putri, SP.P (K), M.biomedNovery SimbolonNo ratings yet
- Kuby Immunology, 7e: Chapter 2: Cells, Organs, and Microenvironments of The Immune SystemDocument37 pagesKuby Immunology, 7e: Chapter 2: Cells, Organs, and Microenvironments of The Immune Systemnemezienna100% (1)
- Induced Pluripotent Stem Cells in Medicine and BiologyDocument5 pagesInduced Pluripotent Stem Cells in Medicine and BiologyMaria Del Mar Robles100% (1)
- Toolkit Biology 01 CellDocument102 pagesToolkit Biology 01 CellargudinmariaNo ratings yet
- CBC (Adult)Document1 pageCBC (Adult)MAYE AMARNo ratings yet
- Developmental Biology GametogenesisDocument14 pagesDevelopmental Biology Gametogenesispragyamaharjan2No ratings yet
- CBC ReportDocument1 pageCBC ReportKamal DeepNo ratings yet
- BD Stemflow StemCell Isolation Analysis Kit PDFDocument2 pagesBD Stemflow StemCell Isolation Analysis Kit PDFapalanavedNo ratings yet
- Hematopoietic Stem CellDocument8 pagesHematopoietic Stem CellMaryam ShahrasbiNo ratings yet
- CHC Jawa Hub: Jawa, Rewa, Madhya Pradesh Rewa Madhya Pradesh - 486223 Phone No.Document2 pagesCHC Jawa Hub: Jawa, Rewa, Madhya Pradesh Rewa Madhya Pradesh - 486223 Phone No.MAHESH GAUTAMNo ratings yet
- Irrigation Policy 2070Document17 pagesIrrigation Policy 2070Gajendra JoshiNo ratings yet
- GENERAL BIOLOGY Cell Types Cell ModificationsDocument4 pagesGENERAL BIOLOGY Cell Types Cell ModificationswenkamuyNo ratings yet
- Blood Anatomy and Physiology ReviewDocument20 pagesBlood Anatomy and Physiology ReviewStacey CamilleNo ratings yet
- WBC CountingDocument4 pagesWBC CountingMarx AsuncionNo ratings yet
- White Blood CorpusclesDocument25 pagesWhite Blood CorpusclesEricaNo ratings yet
- Erythroid PrecursorsDocument1 pageErythroid PrecursorsMezouar AbdennacerNo ratings yet
- CBC-Report RDocument1 pageCBC-Report Ryoutube premiumNo ratings yet
- (Hematology) Chapter 7: Hematopoiesis: BloodDocument5 pages(Hematology) Chapter 7: Hematopoiesis: BloodJean BelciñaNo ratings yet
- Hema 1-Module 2 - Topic 2-Answer SheetDocument3 pagesHema 1-Module 2 - Topic 2-Answer SheetKervy Jay AgraviadorNo ratings yet
- 1 LeukopoiesisDocument23 pages1 LeukopoiesisJan Victor Elico100% (3)
- (Transes) Human Histology - 12 Peripheral BloodDocument5 pages(Transes) Human Histology - 12 Peripheral BloodReina CastronuevoNo ratings yet