Professional Documents
Culture Documents
Aging Imune
Uploaded by
SCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Aging Imune
Uploaded by
SCopyright:
Available Formats
Aging and innate immune cells
Timothy P. Plackett,*,†,‡ Eric D. Boehmer,* Douglas E. Faunce,†,§,¶
and Elizabeth J. Kovacs*,†,§,¶,1
Departments of *Cell Biology, Neurobiology, and Anatomy and ¶Surgery, §Immunology and Aging Program, †Burn
and Shock Trauma Institute, Loyola University Chicago, Maywood, Illinois; and ‡Chicago College of Osteopathic
Medicine, Midwestern University, Downers Grove, Illinois
Abstract: The innate immune system serves an groups between 18 and 59 years of age are considered young.
important role in preventing microbial invasion. The SENIEUR protocol, which designates young as 25–34
However, it experiences significant changes with years old and aged as 65 and above, is not exclusively used as
advancing age. Among the age-associated changes a result of its limitations [8]. The SENIEUR protocol has
are: Aged macrophages and neutrophils have im- extensive exclusion criteria, limiting studies to only the most
paired respiratory burst and reactive nitrogen in- healthy individuals. As a consequence, as much as 85% of the
termediates as a result of altered intracellular sig- aged population is not included by these studies [9, 10]. These
naling, rendering them less able to destroy bacte- subjects are excluded based on the concern that any altered
ria. Aged neutrophils are also less able to respond immune responses may be a result of underlying infection or
to rescue from apoptosis. Aged dendritic cells (DC) pathology; however, it is equally possible that this group has an
are less able to stimulate T and B cells. The altered aging-related, altered immune system that predisposed them to
T cell stimulation is a result of changes in human developing infection or pathology. Given our inability to re-
leukocyte antigen expression and cytokine produc- solve these two possibilities, we have noted when information
tion, and lower B cell stimulation is a result of is based on studies using the strict SENIEUR protocol guide-
changes in DC immune complex binding. Natural lines.
killer (NK) cells from the elderly are less capable of Concerning animal studies, no concise definition of aged has
destroying tumor cells. NK T cells increase in num- been developed. However, Miller and Nadon [11] have outlined
ber and have greater interleukin-4 production with multiple considerations relevant to such a definition; in light of
age. Levels of various complement components are these factors, rodents, aged 16 months and older, are consid-
also altered with advancing age. J. Leukoc. Biol. ered aged for the purposes of this review. Aging studies that
76: 291–299; 2004. specifically used animals with cancer, autoimmune disorders,
or other conditions are excluded from this discussion.
Key Words: immunosenescence 䡠 macrophage 䡠 dendritic cell
䡠 polymorphonuclear neutrophil
POLYMORPHONUCLEAR NEUTROPHILIC
LEUKOCYTES (PMN)
INTRODUCTION
PMN quickly respond to the site of infection and begin to
Clinical data have demonstrated that the ability of the elderly phagocytose opsonized particles. Consequently, they are
to respond to infection is diminished in comparison with young among the first cells to produce bacteriostatic and bacteriocidal
adults. As a result, aged individuals make up a disproportion- products, as well as influence adaptive immunity. As the body’s
ate number of infectious disease patients and are at greater risk first response to microbial invasion and injury, the chemotactic
of contracting more severe and longer lasting infections. In migration, adhesion, and phagocytic capabilities of PMN are
particular, they have higher rates of respiratory tract infections, critical to their proper functioning. In vivo, examination of skin
which are more likely to be caused by atypical organisms [1, 2]. abrasion exudate demonstrated equal migration of PMN into
Moreover, elderly individuals are more prone to developing the site of injury in young and old, although there was a greater
invasive Staphyloccus aureus infections and tetanus [3, 4] and variation in migration amongst the elderly subjects meeting the
have poor responses to influenza vaccination [5, 6]. The aged SENIEUR protocol guidelines compared with the young [12].
population also has a higher incidence of infections and sepsis When young and old volunteers were compared in an experi-
after a traumatic injury than young adults [7]. An age-associ-
ated decline in immunity is implicated as the primary cause of
these problems. This review will examine the effects of aging
1
on the innate immune system, in particular, examining neutro- Correspondence: Department of Cell Biology, Neurobiology, and Anatomy,
phils, dendritic cells (DC), macrophages, natural killer (NK) Department of Surgery, Loyola University Chicago, Stritch School of Medicine,
Building 110, Room 4237, 2160 South First Avenue, Maywood, IL 60513.
cells, NK T (NKT) cells, and complement. E-mail: ekovacs@lumc.edu
For the purposes of this review, any study using a human Received November 25, 2003; accepted February 16, 2004; doi: 10.1189/
population 60 years of age or greater is considered elderly, and jlb.1103592.
Journal of Leukocyte Biology Volume 76, August 2004 291
mental gingivitis model, an equal number of PMN were de- tionally, actin polymerization is significantly diminished after
tected in the junctional epithelium 7 days after injury [13]. In stimulation of PMN from aged subjects with fMLP or PMA,
vitro research has yielded more conflicting results. Corberand relative to young [24, 25]. These age-associated differences in
et al. [14] reported chemotaxis to be significantly decreased in actin correlate with altered cell-surface markers. In particular,
people over the age of 80 years, and there was no significant there is lower surface expression of the activation-inducer
difference in 60- to 70-year-olds compared with young volun- molecule CD69 and chemotactic peptide receptor for N-formyl-
teers. The very opposite was true in a study by Niwa et al. [15], Nle-Leu-Phe,Tyr-Lys hexapeptide on aged PMN after stimula-
which found a correlation between 60- to 70-year-old volun- tion, and no differences are noted in CD11b or complement
teers with diminished PMN chemotaxis and respiratory burst receptor b [25, 26]. These results, taken in their totality,
and their mortality 7 years after the initial study. However, the suggest that the diminished production of ROS may be caused
study demonstrated no difference between people older than 80 partially by impaired intracellular signaling, resulting from an
years old and the young. They suggested that the lack of inability of actin to adequately transport the appropriate cell-
significant difference between the over-80-year-old population surface receptors to the cell membranes of PMN from the aged.
and the youth was a result of a selective pressure that left only Given the short life-span of a PMN, alterations in apoptotic
those individuals with the “healthiest” PMN surviving into the cell death may also predispose the elderly to an increased risk
oldest age group. of infection. In the absence of stimulation, the amount of
Once PMN have migrated to the site of injury or bacterial apoptotic cell death of human PMN is unaltered by aging
entry, they must adhere to the endothelium to diapedis out of [27–29], and there is no difference in the expression of CD95,
the vasculature. Adhesion, however, is not impaired in the APO1/Fas, an apoptotic ligand [30]. In contrast, there are
elderly. When stimulated by f-Met-Leu-Phe (fMLP), opsonized significant variations in apoptosis following cell stimulation.
zymosan, phorbol myristate acetate (PMA), or calcium iono- Under normal conditions, in the young, inflammatory mediators
phore A23187, human PMN obtained from young and aged are able to prevent apoptosis [31–34]. This response to inflam-
subjects adhere equally to plastic, gelatin, and bovine aortic mation helps guarantee the continued involvement of PMN in
endothelium [12, 16]. preventing the spread of microbes, while other cell populations
Phagocytosis is also unimpaired in the elderly. Studies show traffic to the site of injury or insult. However, unlike with the
that the amount of opsonized paraffin oil or yeast cells engulfed young, interleukin (IL)-2, LPS, GM-CSF, or G-CSF do not
is equal in PMN from young and aged volunteers [15, 17]. It is rescue PMN from the elderly [28, 29]. This is paralleled by
interesting that another study with yeast cells showed an in- alterations in the Janus tyrosine kinase (Jak)2-signal trans-
crease in phagocytosis by the elderly in comparison to 20- to ducer and activator of transcription (STAT)5 signaling pathway
29-year-olds; however, no difference between the elderly and in PMN from the elderly. Ultimately, this results in aged PMN
30- to 60-year-olds [14] was noted. These combined results producing increased Bax and decreased Mcl-1, creating a
indicated that there is no impairment in the ability of PMN proapoptotic environment [35].
from aged subjects to traffic to tissues and engulf microbes. The failure of inflammatory mediators to prevent apoptosis in
In contrast to phagocytosis, the microbiocidal capacity of the elderly may also reflect an inability of the cells to prevent
PMN is significantly decreased with advancing age. The ability reactive oxygen-induced damage. Tortorella et al. [27] demon-
of stimulated and unstimulated PMN to kill Candida albicans strated that high levels of superoxide dismutase were better
is attenuated by 10 –50% in the elderly [14, 18], and Esche- able to inhibit apoptosis in PMN from young volunteers than
ricihia coli killing is 44% lower than that of young subjects from aged; however, the addition of catalase raised the apo-
[19]. Contributing to this depressed killing ability is a signif- ptosis inhibition of the aged subjects to a similar level as the
icant decrease in the production of reactive oxygen species young. As inflammatory mediators not only prevent apoptosis
(ROS) by PMN. Following stimulation with fMLP, interferon-␥ but also stimulate the production of ROS in the young [36, 37],
(IFN-␥), granulocyte/monocyte-colony stimulating factor (GM- these results suggest that in an inflammatory environment, the
CSF), or lipopolysaccharide (LPS) [18 –22], the production of PMN from the aged are less able to neutralize free radicals that
superoxide was greatest by PMN from young rather than aged escape from phagolysosomes and will be substantially dam-
humans and rodents. Likewise, nitroblue tetrazolium dye-re- aged. As a result of this damage, these cells are shifted toward
duction tests have yielded higher rates of oxidative dye reduc- a proapoptotic environment and instead undergo programmed
tion by a mixture of young, mature, human neutrophilic gran- cell death.
ulocytes and macrophages [14].
Impaired intracellular signaling has been implicated as a
leading cause for the altered oxygen-free radial production in DC
the aged. Intracellular Ca2⫹ is decreased in stimulated PMN
from elderly volunteers, suggesting that there is an impairment Between a few hours to several days after PMN migration,
in Ca2⫹ flux during cell signaling [18, 23]. Also, actin, which antigen-presenting cells (APCs) enter the site of infection and
may play a role in cell-surface receptor movement and expres- inflammation. DC are the most potent, professional APC of the
sion, has been suggested to contribute to the altered free- immune system [38]. With their ability to interact with B and
radical production. Using actin-stabilizing and -disrupting T cells and their widespread localization, they are a vital
agents, Piazzolla et al. [20] demonstrated that the release of component of the innate immune system.
O2– by PMN from elderly volunteers is more sensitive to these The follicular DC (FDC) is critical to the formation of plasma
agents, yielding significantly lower superoxide release. Addi- cells in the germinal centers of secondary lymphoid organs and
292 Journal of Leukocyte Biology Volume 76, August 2004 http://www.jleukbio.org
the subsequent generation of antibodies [39]. Szakal and co- MACROPHAGES
workers [40, 41] reported that decreases in the cell-surface
receptors for complement, CD21, and Fc␥ in aged mice im- Macrophages play important roles in the immune response,
paired the initial trapping of immune complexes by FDC. This capable of not only phagocytosis and destruction of foreign
is further complicated by a decrease in the number of icco- cells and debris but also acting as APCs. Like DC, they stand
somes, antigen:antibody complexes that bud off FDC and are at the crossroads between adaptive and innate immunity.
taken up by B cells [40]. The combined effect is less-stable Macrophages are able to directly destroy microbes through
binding and decreased stimulation of B cells. Deficient anti- the products of the respiratory burst and reactive nitrogen-
body production can be partially restored in vitro by using FDC intermediate pathways. Both of these processes are induced by
that have a higher percentage of immune complexes and com- IFN-␥ from T cells and NK cells or by components of bacterial
plement bound [40, 41]. In addition, FDC of aged mice often cell walls [54, 55]. The binding of IFN-␥ or bacterial products
remained trapped in the subcapsular sinus of lymph nodes and to their appropriate receptors results in phosphorylation of
are unable to reach the follicle and its B cells, resulting in mitogen-activated protein kinase (MAPK) and the subsequent
decreased germinal center formation [40, 42]. These changes release of free radicals. Studies using rats have demonstrated a
in FDC are partially responsible for the significant modification 75% decrease in the ability of macrophages from aged animals
of humoral immunity in the elderly [43]. to produce superoxide anion following incubation with IFN-␥
Non-FDC, hereafter referred to simply as DC, are capable of or opsonized zymosan [56]. The impaired superoxide produc-
activating CD4⫹ T cells via their major histocompatibility tion by macrophages from the aged was further investigated by
complex class II (MHC-II) [44]. DC from young and aged Ding et al. [57], who demonstrated that caseinate-elicited
humans induce similar amounts of T cell proliferation and have peritoneal macrophages from aged mice had undetectable
similar levels of human leukocyte antigen (HLA) expression phosphorylation of MAPK following incubation of the cells with
[45, 46]. However, lower HLA-DR expression has also been IFN-␥. Functionally, this abrogation in MAPK activation was
reported [47]. In the former studies, DC were obtained by correlated with 50% less hydrogen peroxide production follow-
stimulating monocytes with GM-CSF, and the latter study ing stimulation of macrophages from aged mice with PMA or
analyzed peripheral blood DC. Differences in these protocols opsonized zymosan. Others [58 – 60] have also reported a sim-
may account for the opposing results and suggest that there is ilar decrease in oxygen free-radial production by macrophages
a need for careful interpretation of ex vivo experiments. These from aged mice. As a consequence of reduced respiratory burst
different results also suggest that although circulating DC from in the elderly, the intercellular killing of bacteria is hindered
the elderly are less prepared to bind antigens, the deficiency and thus may cause the elderly to have infections of longer
can be corrected in the appropriate environment. Animal mod- duration [61– 63].
els have also encountered conflicting information. T cell pro- The production of reactive nitrogen intermediates is the
liferation was attenuated when cultured with DC from aged other key mechanism by which macrophages exert their mi-
DBA or A mice, and there was no difference with BALB/c, crobicidal properties; however, studies into the production of
CBA, or C3H/He mouse DC [48]. Reasons for these differences nitrogen compounds in the aged offer conflicting results. Sev-
remain to be explored. Aside from T cell proliferation, aged, eral reports demonstrated that inducible nitric oxide synthase
murine DC are also less able to stimulate peripheral blood (iNOS) mRNA is decreased in aged mice [64, 65]. Addition-
mononuclear cell (PBMC) production of IFN-␥, further con- ally, in the absence of iNOS, aged mice [64 – 66] significantly
tributing to the changes in T cell functionality with advancing decreased the production of NO2– and other intermediates.
age [49]. Conversely, enhanced iNOS mRNA and nitrite production by
Within the peripheral blood of the elderly, there is nearly macrophages of the aged have also been reported. Chen et al.
50% less CD11c⫹B220⫹ plasmacytoid DC [50]. However, it is [67] demonstrated an increase in nitrite production in resident
unknown if there are differences in the CD11c⫹B220–CD8⫹ and thioglycollate-elicited peritoneal macrophages of aged
lymphoid or CD11c⫹B220–CD8– myeloid DC subsets with mice after LPS stimulation, as well as increased iNOS mRNA
advancing age. This decrease in DC exists despite an increase in thioglycollate-elicited peritoneal macrophages. Others [68]
in the proliferation of peripheral DC from the elderly after in have also reported similar increases. The conflicting reports
vivo GM-CSF incubation when compared with the young [45]. are likely a result of differences in experimental protocols.
The proliferation studies, however, use the SENIEUR protocol Recently, it was demonstrated that thioglycollate-elicited mac-
and did not account for age-related differences in circulating rophages stimulated with LPS and IFN-␥ display different
GM-CSF, whose levels are lower in aged animals; therefore, age-specific nitrite production patterns based on the dose of
stimulating PBMC from young and aged subjects with equiv- IFN-␥ with which the cells were cultured [69]. At low doses of
alent concentrations of GM-CSF does not accurately reflect the IFN-␥, the production of nitrite was higher in young mice than
natural microenviroment [51, 52]. The lower circulating GM- in old mice. However, at a higher dose, the macrophage from
CSF of the elderly may result in less DC proliferation. Addi- older mice had a greater production of nitrite than the young
tionally, it has been noted that in vitro and in vivo models of mice. Therefore, interpretation and application of the results
DC function have resulted in divergent results [53]. Conse- require a consideration of the microenvironment in which the
quently, although there is a decrease in absolute number of cells function.
DC, further research is needed to determine if DC retained Macrophages from the aged can also contribute to the dys-
their proliferative capacity with advancing age and what effects regulation seen in other immune cell populations (Fig. 1). The
lower circulating GM-CSF levels may have on DC function. production of PGE2 by macrophages is enhanced in aged mice,
Plackett et al. Innate immunity in aging 293
mine which cell population is responsible for potential height-
ened inflammatory states.
Wound healing is dependent on macrophage function, as
macrophages are able to keep the wound bed free from infec-
tion and promote angiogenesis [92]. With its dual roles, mac-
rophage migration to the site of injury can be critical in the
post-injury immune response. After injury, chemokines, in-
cluding monocyte chemoattractant protein-1 (MCP-1), are pro-
duced to promote the migration of monocytes and macrophages
into the wound bed. Following excisional wounding, macro-
phages begin to infiltrate into the wound bed within 24 h [93].
The infiltration is significantly greater in aged mice and cor-
relates with increased MCP-1 in wound homogenates 12 h after
injury, relative to young mice. However, burn injury is associ-
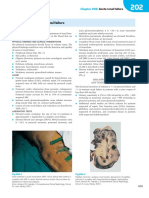
Fig. 1. Changes in macrophage signaling, enzymatic processes, and output ated with decreased MCP-1 by aged, injured mice, relative to
with advancing age. Arrows (1 and 2) indicate increased or decreased levels young, injured mice, 24 h after injury. However there was
in the elderly compared with young. TNF-␣, Tumor necrosis factor ␣; JNK, equal macrophage accumulation in the region immediately
c-jun NH2-terminal kinase; TLR4, Toll-like receptor 4; COX, cyclooxygenase; adjacent to the necrotic, burn-injured tissue in young and aged,
PGE2, prostaglandin E2.
injured mice [94]. The differences between the results of these
two studies may reflect variations in the experiment models.
Burn injury is associated with significant increases in proin-
which correlates with an increase in COX-2 mRNA and en- flammatory cytokines in aged mice [85], which may alter
zyme levels [70]. PGE2 is able to alter DC function by blocking macrophage migration. Studies of excisional wound healing in
the production of IL-12, increasing production of IL-10, and humans demonstrate a delay in monocyte and macrophage
decreasing MHC-II expression in mice [71–73]. It also exerts infiltration with age [95]. The slow infiltration is associated
effects on T cells. The arachidonic acid metabolite is capable with a decrease in intercellular adhesion molecule-1 and vas-
of decreasing IL-2 production and subsequently lowering T cular adhesion molecule-1 expression, suggesting an impair-
cell-proliferative responses [74, 75], two well-documented ment in the ability of the cells to bind to the endothelium to
changes seen with advancing age [76 –78]. Up-regulation of T leave the circulation [95].
helper cell type 2 (Th2) cytokines, which are known to be Once at the site of injury, macrophages can promote angio-
elevated in elderly human patients and in animal models of genesis and wound healing through the production of vascular
aging [76, 79, 80], is also a result of mouse splenocyte stim- endothelial growth factor (VEGF). This angiogenic mediator is
ulation with PGE2 [74]. present at lower levels in excisional wounds from aged mice,
Macrophage-derived cytokines also affect the immune re- which correlates with 37% less VEGF production by stimu-
sponse. Several recent reports have suggested that there is a lated peritoneal macrophages from aged mice [96]. The dimin-
decrease in the production of proinflammatory cytokines by ished VEGF production and angiogenesis may delay wound
macrophages from aged humans and mice. Renshaw et al. [81] closure, allowing more time for bacteria to circumvent the
showed that LPS stimulation of splenic and thioglycollate- natural skin barrier and develop into a full infection. With only
elicited peritoneal macrophages resulted in less IL-6 and isolated studies exploring the role of macrophages in wound
TNF-␣ secretion by macrophages from aged mice compared healing of the elderly, further research is needed before defin-
with young mice. Furthermore, the study suggested that lower itive conclusions can be reached.
proinflammatory cytokine differences may be a result of lower
TLR4 mRNA in macrophages of aged mice, rendering the
macrophages less responsive to LPS. Lower IL-6 and TNF-␣ NK CELLS
production by LPS-stimulated macrophages is also associated
with decreased, activated p38 and JNK MAPKs [82]. Beharka Although PMN, DC, and macrophages are capable of seeking
et al. [83] demonstrated a decrease in the production of IL-6 by out and eliminating extracellular pathogens, NK cells are crit-
human PBMC cultured with autologous plasma. Most litera- ical to removal of intracellular pathogens. In addition to their
ture, however, suggests that circulating levels of proinflamma- importance in viral infections, they also possess vital tumori-
tory cytokines are elevated in the aged [84 – 87]. Macrophages cidal activities.
have been assumed to be the primary producer of these cyto- There is an increase in the relative percentage of NK cells
kines. Several studies have demonstrated that in fact, under- with advancing age when comparing SENIEUR protocol-qual-
lying inflammatory disease and poor nutrition may actually be ified elderly and young individuals [97–99]. The rise in per-
responsible for this circulating, proinflammatory state, rather centage of NK cells in the elderly is related to a decrease in the
than the natural aging process [88 –91]. Also, although mac- number of T cells and an increase in the absolute number of
rophages have been assumed to be a primary producer of the NK cells [30, 97, 100 –102]. Aside from an increase in number,
proinflammatory cytokines in the elderly, other cell popula- NK cells obtained from the aged are more likely to have a
tions may contribute to cytokine production as well. In light of mature phenotype, having a higher percentage and absolute
the more recent findings, further research is needed to deter- number of CD56dim cells and a smaller CD56bright population
294 Journal of Leukocyte Biology Volume 76, August 2004 http://www.jleukbio.org
[100, 103]. Additionally, NK cells from the aged may be less injury responses [120], microbial clearance [121], autoimmune
prepared to carryout their prescribed function, as there is a disease [122], and other immune responses [123, 124].
significant increase in the number of agranular cells in humans Absolute numbers and relative percentages of NKT cells
[97] and a decrease in the adherence to tumor cells in mice increase in number with advancing age. The increase in NKT
[104]. cells appears to be a global rise, as increases of 150 – 400%
Conflicting reports exist as to the cytolytic ability of NK cells have been reported in circulating, splenic, hepatic, mesenteric
in the elderly. Independent studies demonstrated that NK cells lymph node, and peripheral lymph node counts in humans and
in the elderly have normal [105] or enhanced activity [106] rodents [125–128]. Cytokine production by NKT cells has also
compared with young individuals when the elderly population varied with aging. Dubey et al. [129] showed an increase in
is selected based on strict adherence to the SENIEUR protocol. production of IL-4 mRNA and protein by CD4⫹ NKT cells
However, when elderly patients were selected for good health from aged B.10 mice and a concurrent decrease in IFN-␥
but did not fully meet the SENIEUR protocol, the NK cell mRNA and protein after stimulation of the cells with ␣CD3 and
activity against tumor cells was significantly decreased com- IL-2; however, the aged group included mice as young as 12
pared with young adults. Other human studies, which more months old [129]. In contrast, Poynter et al. [128] found that
loosely followed the suggestion of the SENIEUR protocol, also ␣CD3 stimulation of NKT cells leads to lower IL-4 and IL-2
demonstrated an age-specific decrease in NK cell cytotoxicity mRNA in aged C57BL/6 mice. Although both studies con-
toward tumor cells [98, 107, 108], and similar results in animal cluded that there is a decrease in Th1 cytokine mRNA, the
models used LPS or concanavalin A as cell stimulants [109 – discrepancy in IL-4 remains to be resolved.
112]. These reports indicate that although fully functional NK Expression of Fas ligand following cell stimulation displays
cells may be desirable for optimal health, the majority of the an age-related effect as well. Younger mice express lower
elderly population will experience some deficit in NK cell levels of Fas ligand after injection of ␣-GalCer into the mice
activity. This also highlights the need for careful interpretation [130]. Studies with young Fas-deficient and wild-type mice
and application of SENIEUR protocol-based information. have demonstrated that injection of ␣-GalCer results in an
Alterations in intracellular signaling are key contributors to increase in NKT cell apoptosis and that the cell death is
the tumoricidal deficiency [102]. In particular, there is a delay Fas-dependent [131]. However, whether the increased Fas
in phosphatidlyinositol biphosphate hydrolysis, coupled with a ligand in aged mice exerts an effect on NKT apoptosis is
failure of inositol triphosphate to increase above basal levels unknown.
[102]. However, these results were specific to spontaneous
cytolytic activity directed toward tumor cells, as antibody-
dependent, cell-mediated cytotoxicity (ADCC), assayed by an-
ti-CD16 antibody stimulation of the Fc receptor, was intact in COMPLEMENT
the aged subjects. Likewise, the production of insositol phos-
phate activity was comparable with the young during ADCC. Most studies examining the relationship of age and comple-
Others have also reported an intact ADCC [113, 114]. The ment focused on the role of C1q in Alzheimer’s disease [132–
impaired, spontaneous cytolytic activity allows for continued 134]; however, a limited number of studies compare the levels
growth and expansion of neoplastic cells and is likely a con- of complement proteins and their functionality in the young
tributor to the increased incidence of cancer among the elderly and aged. When considering the classical complement path-
[115, 116]. way, serum levels of C4 were increased in aged volunteers
In addition to decreased toxicity, the NK cells from the [135–137] or unchanged between the young and old [138].
elderly are unable to properly proliferate following IL-2 stim- These differences in C4 protein may reflect the use of the
ulation. In human- and murine-derived NK cells, the prolifer- SENIEUR protocol by Bellavia et al. [138]. In an isolated
ative response to IL-2 is decreased by 40 – 60% among the study, there was an increase in the plasma levels of C4-binding
elderly [100, 104]. The decreased, proliferative response is protein (C4BP) with age [139]. However, interpretation of this
paralleled by an age-specific decrease in Ca2⫹ mobilization datum is limited, as the study does not state which age groups
[100]. The diminished Ca2⫹ prevented the up-regulation of were compared. The role that the age-associated increase in
CD69 on elderly human NK cells, an early activator of NK cell C4BP has on immunity is also uncertain, as the increase is
proliferation and cytotoxicity. The combined effect of dimin- attributed to a rise in C4BP. The C4BP subunit interacts
ished proliferation responses and cytotoxic activity of NK cells with the coagulation cascade, whereas C4BP␣ specifically
is that the elderly are more prone to developing longer lasting binds to C4b and inhibits complement activation [140]. Using
infections and a decreased ability to rid the body of tumor cells a rat model, Lavery and Goyns [141] described a decrease in
[117, 118]. the expression of the C4BP␣ in the livers of aged animals;
however, it is unknown whether this observation is organ- or
species-specific.
Description of age-related changes in the alternative com-
NKT CELLS plement pathway has also yielded conflicting results. Serum C3
levels were reported to be increased [135, 137] in the elderly
NKT cells are a minor cell population found in most lymphoid or equivalent in young and aged [136, 138]. Additionally,
organs. They are characterized as CD1d-restricted cells [119] Factor B levels in elderly humans are either decreased [135] or
and have been implicated as a significant contributor to post- not significantly different from the young [136].
Plackett et al. Innate immunity in aging 295
TABLE 1. Significant Changes with Advancing Age tions between DC and macrophages with T and B cells. Thus,
the effect of aging on innate immunity is not merely an increase
Cell population Changes with aging References in relative percentage of innate immune cells, but it is a
Neutrophils 2 Reactive oxygen species [19, 20, 22] dynamic system reflecting vast changes in all of its many
1 Apoptosis [33, 35] components.
Dendritic cells 2 B cell stimulation [40, 43]
2 Number of plasmacytoid DC [50]
Macrophages 2 Reactive oxygen species [56, 60]
2 Reactive nitrogen intermediates [65] ACKNOWLEDGMENTS
1 Prostaglandin E2 [70]
2 Interleukin-6 [81, 82]
Natural killer We thank Dr. Pamela Witte, Director of the Immunology and
cells 1 Number of NK cells [30] Aging Program (Loyola University, Maywood, IL), for her crit-
2 Tumor-killing activity [108, 109] ical review of the manuscript and thoughtful discussion. NIH
Natural killer T R01 AG18859 supported this work.
cells 1 Number of NKT cells [125, 126]
REFERENCES
As with the components unique to the classic and alternative
complement pathways, research into the common pathway 1. Gotfried, M. H. (2001) Epidemiology of clinically diagnosed community-
components is also extremely limited and fraught with contra- acquired pneumonia in the primary care setting: results from the 1999 –
dictions. Cannon et al. [142] found equivalent rises of C3a 2000 respiratory surveillance program. Am. J. Med. 111, 25S–29S.
2. Ruiz, M., Santiago, E., Marcos, M. A., Martinez, J. A., Arancibia, F.,
anaphylatoxin in plasma obtained from young and old humans Mensa, J., Torres, A. (1999) Etiology of community-acquired pneumonia:
after exercise-induced tissue damage, whereas Kyrkanides et impact of age, comorbidity, and severity. Am. J. Respir. Crit. Care Med.
al. [143] showed an increase in C3a1 mRNA in aged mice after 160, 397– 405.
3. Laupland, K. B., Church, D. L., Mucenski, M., Sutherland, L. R., Davies,
a cortical stab injury. The ultimate significance of either of H. D. (2003) Population-based study on the epidemiology of and the risk
these results remains to be demonstrated. factors for invasive Staphylococcus aureus infections. J. Infect. Dis. 187,
Functional studies of complement activity indicate normal 1452–1459.
4. Pascual, F. B., McFinley, E. L., Zanardi, L. R., Cortese, M. M., Murphy,
functioning or a decrease in activity with advancing age. Haz- T. V. (2003) Tetanus surveillance–United States, 1998 –2000. MMWR
lett et al. [144] reported a 50% decrease in alternative path- Surveill. Summ. 52, 1– 8.
way-induced hemolysis by aged Swiss ICR mice. The impaired, 5. Bridges, C. B., Harper, S. A., Fukuda, K., Uyeki, T. M., Cox, N. J.,
Singleton, J. A., Advisory Committee on Immunization Practices. (2003)
alternative pathway was also associated with a decrease in the Prevention and control of influenza. Recommendations of the Advisory
number of PMN undergoing phagocytosis of Pseudomonas Committee on Immunization Practices (ACIP). MMWR Recomm. Rep.
aeruginosa. However, there was no difference in the total 52, 1–34.
6. Potter, J. M., O’Donnell, B., Carman, W. F., Roberts, M. A., Stott, D. J.
amount of bacteria remaining after the incubation. Using the (1999) Serological response to influenza vaccination and nutritional and
SENIEUR protocol, Bellavia et al. [138] found no difference in functional status of patients in geriatric medical long-term care. Age
the ability of the alternative or classical pathway to induce Ageing 28, 141–145.
7. Linn, B. S. (1980) Age differences in the severity and outcomes of burns.
hemolysis in aged versus young individuals. Given the limited J. Am. Geriatr. Soc. 28, 118 –123.
number of studies examining complement functionality, further 8. Ligthart, G. J., Corberand, J. X., Fournier, C., Galanaud, P., Hijmans,
research is needed before definitive conclusions can be drawn. W., Kennes, B., Muller-Hermelink, H. K., Steinmann, G. G. (1984)
Admission criteria for immunogerontological studies in man: the
SENIEUR protocol. Mech. Ageing Dev. 28, 47–55.
9. Ligthart, G. J., Corberand, J. X., Geertzen, H. G., Meinders, A. E.,
CONCLUSION Knook, D. L., Hijmans, W. (1990) Necessity of the assessment of health
status in human immunogerontological studies: evaluation of the
SENIEUR protocol. Mech. Ageing Dev. 55, 89 –105.
This review has provided an in-depth examination of the cur- 10. Traill, K. N., Schonitzer, D., Jurgens, G., Bock, G., Pfeilschifter, R.,
rent knowledge concerning aging and its effects on innate Hilchenbach, M., Holasek, A., Forster, O., Wick, G. (1985) Age-related
changes in lymphocyte subset proportions, surface differentiation antigen
immunity. Contrary to prior beliefs, the innate immune system density and plasma membrane fluidity: application of the Eurage
is not free from the effects of aging. Although the age-associ- SENIEUR protocol admission criteria. Mech. Ageing Dev. 33, 39 – 66.
ated decrease in T and B cell populations allows for an in- 11. Miller, R. A., Nadon, N. L. (2000) Principles of animal use for geronto-
logical research. J. Gerontol. A Biol. Sci. Med. Sci. 55, B117–B123.
crease in the percentage of innate cell, like their adaptive cell 12. Biasi, D., Carletto, A., Dell’Agnola, C., Caramaschi, P., Montesanti, F.,
counterparts, the innate cells do not function normally (Table Zavateri, G., Zeminian, S., Bellavite, P., Bambara, L. M. (1996) Neutro-
1). The production of ROS and nitrogen species is significantly phil migration, oxidative metabolism, and adhesion in elderly and young
subjects. Inflammation 20, 673– 681.
impaired in neutrophils and macrophages from the aged, pos- 13. Fransson, C., Mooney, J., Kinane, D. F., Berglundh, T. (1999) Differ-
sibly affecting bacterial destruction, and NK cells are less able ences in the inflammatory response in young and old human subjects
to destroy tumor cells. There is a significant increase in the during the course of experimental gingivitis. J. Clin. Periodontol. 26,
453– 460.
number of NKT cells in the aged, but further research is 14. Corberand, J., Ngyen, F., Laharrague, P., Fontanilles, A. M., Gleyzes, B.,
needed to determine the functional significance. Likewise, Gyrard, E., Senegas, C. (1981) Polymorphonuclear functions and aging in
complement research remains a vastly understudied area of humans. J. Am. Geriatr. Soc. 29, 391–397.
15. Niwa, Y., Kasama, T., Miyachi, Y., Kanoh, T. (1989) Neutrophil chemo-
aging research. Finally, the innate immune system also con- taxis, phagocytosis and parameters of reactive oxygen species in human
tributes to altered acquired immunity with aging, via interac- aging: cross-sectional and longitudinal studies. Life Sci. 44, 1655–1664.
296 Journal of Leukocyte Biology Volume 76, August 2004 http://www.jleukbio.org
16. Damtew, B., Spagnuolo, P. J., Goldsmith, G. G., Marino, J. A. (1990) processing machinery during dendritic cell maturation. Int. Immunol.
Neutrophil adhesion in the elderly: inhibitory effects of plasma from 13, 1515–1523.
elderly patients. Clin. Immunol. Immunopathol. 54, 247–255. 39. Zhang, X., Li, L., Jung, J., Xiang, S., Hollmann, C., Choi, Y. S. (2001)
17. Fulop, T., Foris, G., Leovey, A. (1984) Age-related changes in cAMP and The distinct roles of T cell-derived cytokines and a novel follicular
cGMP levels during phagocytosis in human polymorphonuclear leuko- dendritic cell-signaling molecule 8D6 in germinal center-B cell differ-
cytes. Mech. Ageing Dev. 27, 233–237. entiation. J. Immunol. 167, 49 –56.
18. Seres, I., Csongor, J., Mohacsi, A., Leovey, A., Fulop, T. (1993) Age- 40. Szakal, A. K., Taylor, J. K., Smith, J. P., Kosco, M. H., Burton, G. F.,
dependent alterations of human recombinant GM-CSF effects on human Tew, J. J. (1990) Kinetics of germinal center development in lymph nodes
granulocytes. Mech. Ageing Dev. 71, 143–154. of young and aging immune mice. Anat. Rec. 227, 475– 485.
19. Fu, Y-K., Arkins, S., Li, Y. M., Dantzer, R., Kelley, K. W. (1994) 41. Aydar, Y., Balogh, P., Tew, J. G., Szakal, A. K. (2002) Age-related
Reduction in superoxide anion secretion and bactericidal activity of depression of FDC accessory functions and CD21 ligand-mediated repair
neutrophils from aged rats: reversal by combination of ␥ interferon and of co-stimulation. Eur. J. Immunol. 32, 2817–2826.
growth hormone. Infect. Immun. 62, 1– 8. 42. Holmes, K. L., Schnizlein, C. T., Perkins, E. H., Tew, J. G. (1984) The
20. Piazzolla, G., Tortorella, C., Serrone, M., Jirillo, E., Antonaci, S. (1998) effect of age on antigen retention in lymphoid follicles and in collagenous
Modulation of cytoskeleton assembly capacity and oxidative response in tissue of mice. Mech. Ageing Dev. 25, 243–255.
aged neutrophils. Immunopharmacol. Immunotoxicol. 20, 251–266. 43. Szakal, A. K., Aydar, Y., Balogh, P., Tew, J. G. (2002) Molecular
21. Tortorella, C., Ottolenghi, A., Pugliese, P., Jirillo, E., Antonaci, S. (1993) interactions of FDCs with B cells in aging. Semin. Immunol 14, 267–
Relationship between respiratory burst and adhesiveness capacity in 274.
elderly polymorphonuclear cells. Mech. Ageing Dev. 69, 53– 63. 44. Granucci, F., Feau, S., Zanoni, I., Pavelka, N., Vizzardelli, C., Raimondi,
22. Tortorella, C., Polignano, A., Piazzolla, G., Serrone, M., Jirillo, E., G., Ricciardi-Castagnoli, P. (2003) The immune response is initiated by
Antonaci, S. (1996) Lipopolysaccharide-, granulocyte-monocyte colony dendritic cells via interaction with microorganisms and interleukin-2
stimulating factor- and pentoxifylline-mediated effects on formyl-methio- production. J. Infect. Dis. 187, S346 –S350.
nyl-leucine-phenylalanine-stimuated neutrophil respiratory burst in the 45. Steger, M. M., Maczek, C., Grubeck-Loebenstein, B. (1996) Morpholog-
elderly. Microbios 85, 189 –198. ically and functionally intact dendritic cells can be derived from the
23. Fulop, T., Varga, Z., Jacob, M. P., Robert, L. (1997) Effect of lithium on peripheral blood of aged individuals. Clin. Exp. Immunol. 105, 544 –
superoxide production and intracellular free calcium mobilization in 550.
elastin peptide (-elastin) and FMLP stimulated human PMNs. Effect of 46. Lung, T. L., Saurwein-Teissl, M., Parson, W., Schonitzer, D., Grubeck-
age. Life Sci. 60, 325–332. Loebenstein, B. (2000) Unimpaired dendritic cells can be derived from
24. Rao, K. M. K. (1986) Age-related decline in ligand-induced actin poly- monocytes in old age and can mobilize residual function in senescent T
merization in human leukocytes and platelets. J. Gerontol. 41, 561–566. cells. Vaccine 18, 1606 –1612.
25. Rao, K. M. K., Currie, M. S., Padmanabhan, J., Cohen, H. J. (1992) 47. Pietschmann, P., Hahn, P., Kudlacek, S., Thomas, R., Peterlik, M. (2000)
Age-related alteration in actin cytoskeleton and receptor expression in Surface markers and transendothelial migration of dendritic cells from
human leukocytes. J. Gerontol. 47, B37–B44. elderly subjects. Exp. Gerontol. 35, 213–214.
48. Komatsubara, S., Cinader, B., Muramatsu, S. (1986) Polymorphism of
26. Noble, J. M., Ford, G. A., Thomas, T. H. (1999) Effect of aging on CD11b
age-related changes in stimulatory capacity of murine dendritic cells.
and CD69 surface expression by vesicular insertion in human polymor-
Mech. Ageing Dev. 37, 163–173.
phonuclear leukocytes. Clin. Sci. 97, 323–329.
49. Looney, R. J., Falsey, A. R., Walsh, E., Campbell, D. (2002) Effects of
27. Tortorella, C., Piazzolla, G., Spaccavento, F., Jirillo, E., Antonaci, S.
aging on cytokine production in response to respiratory syncytial virus
(1999) Age-related effects of oxidative metabolism and cyclic AMP
infection. J. Infect. Dis. 185, 682– 685.
signaling on neutrophil apoptosis. Mech. Ageing Dev. 110, 195–205.
50. Shodell, M., Siegal, F. P. (2002) Circulating, interferon-producting plas-
28. Tortorella, C., Piazzolla, G., Spaccavento, F., Pece, S., Jirillo, E., An-
macytoid dentritic cells decline during human ageing. Scand. J. Immu-
tonaci, S. (1998) Spontaneous and Fas-induced apoptotic cell death in
nol. 56, 518 –521.
aged neutrophils. J. Clin. Immunol. 18, 321–329.
51. Gon, Y., Hashimoto, S., Hayashi, S., Koura, T., Matsumoto, K., Horie, T.
29. Fulop, T., Fouquet, C., Allaire, P., Perrin, N., Lacombe, G., Stankova, J., (1996) Lower serum concentrations of cytokines in elderly patients with
Rola-Pleszczynski, M., Gagne, D., Wagner, J. R., Khalil, A., Dupuis, G. pneumonia and the impaired production of cytokines by peripheral blood
(1997) Changes in apoptosis of human polymorphonuclear granulocytes monocytes in the elderly. Clin. Exp. Immunol. 106, 120 –126.
with aging. Mech. Ageing Dev. 96, 15–34. 52. Cai, N. S., Li, D. D., Cheung, H. T., Richardson, A. (1990) The expres-
30. Di Lorenzo, G., Balistreri, C. R., Candore, G., Cigna, D., Colombo, A., sion of granulocyte/macrophage colony-stimulating factor in activated
Romano, G. C., Colucci, A. T., Gervasi, F., Listi, F., Potestio, M., Caruso, mouse lymphocytes declines with age. Cell. Immunol. 130, 311–319.
C. (1999) Granulocyte and natural killer activity in the elderly. Mech. 53. Grewe, M. (2001) Chronological ageing and photoageing of dendritic
Ageing Dev. 108, 25–38. cells. Clin. Exp. Dermatol. 26, 608 – 612.
31. Colotta, F., Re, F., Polentarutti, N., Sozzani, S., Mantovani, A. (1992) 54. Bogdan, C., Nathan, C. (1993) Modulation of macrophage function by
Modulation of granulocyte survival and programmed cell death by cyto- transforming growth factor , interleukin-4, and interleukin-10. Ann.
kines and bacterial products. Blood 80, 2012–2020. N. Y. Acad. Sci. 685, 713–739.
32. Brach, M. A., de Vos, S., Gruss, H. J., Herrmann, F. (1992) Prolongation 55. Crawford, R. M., Leiby, D. A., Green, S. J., Nacy, C. A., Fortier, A. H.,
of survival of human neutrophils by granulocyte-macrophage colony- Meltzer, M. S. (1994) Macrophage activation: a riddle of immunological
stimulating factor is caused by inhibition of programmed cell death. resistance. Immunol. Ser. 60, 29 – 46.
Blood 80, 2920 –2924. 56. Davila, D. R., Edwards III, C. K., Arkins, S., Simon, J., Kelley, K. W.
33. Pericle, F., Liu, J. H., Diaz, J. I., Blanchard, D. K., Wei, S., Forni, G., (1990) Interferon-␥-induced priming for secretion of superoxide anion
Djeu, J. Y. (1994) Interleukin-2 prevention of apoptosis in human and tumor-necrosis factor-␣ declines in macrophages from aged rats.
neutrophils. Eur. J. Immunol. 24, 440 – 444. FASEB J. 4, 2906 –2911.
34. Lee, A., Whyte, M. K. B., Haslett, C. (1993) Inhibition of apoptosis and 57. Ding, A., Hwang, S., Schwab, R. (1994) Effect of aging on murine
prolongation of neutrophil functional longevity by inflammatory media- macrophages. Dimished response to IFN-␥ for enhanced oxidative me-
tors. J. Leukoc. Biol 54, 283–288. tabolism. J. Immunol. 153, 2146 –2152.
35. Fulop, T., Larbi, A., Linteau, A., Desgeorges, S., Douziech, N. (2002) 58. Hayakawa, H., Sato, A., Yagi, T., Uchiyama, H., Ide, K., Nakano, M.
The role of Mcl-1 and Bax expression alteration in the decreased rescue (1995) Superoxide generation by alveolar macrophages from aged rats:
of human neutrophils from apoptosis by GM-CSF with aging. Ann. N. Y. improvement by in vitro treatment with IFN-␥. Mech. Ageing Dev. 80,
Acad. Sci. 973, 305–308. 199 –211.
36. Power, C., Wang, J. H., Sookhai, S., Wu, Q. D., Redmond, H. P. (2001) 59. Lavie, L., Weinreb, O., Gershon, D. (1992) Age-related alterations in
Proinflammatory effects of bacterial lipoprotein on human neutrophil superoxide anion generation in mouse peritoneal macrophages studied by
activation status, function, and cytotoxic potential in vitro. Shock 15, repeated stimulation and heat shock treatment. J. Cell. Physiol. 152,
461– 466. 382–388.
37. Mansfield, P. J., Minkovska-Galcheva, V., Carey, S. S., Shayman, J. A., 60. Alvarez, E., Machado, A., Sobrino, F., Santa Maria, C. (1996) Nitric
Boxer, L. A. (2002) Regulation of polymorphonuclear leukocyte degran- oxide and superoxide anion production decreases with age in resident
ulation and oxide production by ceramide through inhibition of phospho- and activated rat peritoneal macrophages. Cell. Immunol. 169, 152–
lipase D. Blood 99, 1434 –1441. 155.
38. Li, J., Schuler-Thurner, B., Schuler, G., Huber, C., Seliger, B. (2001) 61. Shepherd, V. L. (1986) The role of the respiratory burst of phagocytes in
Bipartite regulation of different components of the MHC class I antigen- host defense. Semin. Respir. Infect. 1, 99 –106.
Plackett et al. Innate immunity in aging 297
62. Johnston Jr., R. B., Kitagawa, S., Edwards III, C. K., Channon, J. Y., 86. Saurwein-Teissl, M., Blasko, I., Zisterer, K., Neuman, B., Lang, B.,
Suzuki, H., Pabst, M. J. (1988) The respiratory burst in activated mac- Grubeck-Loebenstein, B. (2000) An imbalance between pro- and anti-
rophages: studies of its molecular basis and evidence for downregulation inflammatory cytokines, a characteristic feature of old age. Cytokine 12,
in chronic infection. Adv. Exp. Med. Biol. 239, 63–72. 1160 –1161.
63. Kumar, P., Pai, K., Pandey, H., Sundar, S. (2002) NADH-oxidase, 87. Franceschi, C., Bonafe, M., Valensin, S., Olivieri, F., De Luca, M.,
NADPH-oxidase and myeloperoxidase activity of visceral leishmaniasis Ottaviani, E., De Benedictis, G. (2000) Inflamm-aging: an evolutionary
patients. J. Med. Microbiol. 51, 832– 836. perspective on immunosenescence. Ann. N. Y. Acad. Sci. 908, 244 –254.
64. Khare, V., Sodhi, A., Singh, S. M. (1996 –1997) Effects of aging on the 88. Ahluwalia, N., Mastro, A. M., Ball, R., Miles, M. P., Rajendra, R.,
tumoricidal functions of muring peritoneal macrophages. Nat. Immun. Handte, G. (2001) Cytokine production by stimulated mononuclear cells
15, 285–296. did not change with aging in apparently healthy, well-nourished women.
65. Lu, Q., Ceddia, M. A., Price, E. A., Ye, S-M., Woods, J. A. (1999) Mech. Ageing Dev. 122, 1269 –1279.
Chronic exercise increases macrophage-mediated tumor cytolysis in 89. Chandra, R. K. (1997) Nutrition and the immune system: an introduction.
young and aged mice. Am. J. Physiol. 276, R482–R489. Am. J. Clin. Nutr. 66, 460S– 463S.
66. Kissen, E., Tomasi, M., McCartney-Francis, N., Gibbs, C. L., Smith, 90. Lesourd, B. M. (1997) Nutrition and immunity in the elderly: modifica-
P. D. (1997) Age-related decline in murine macrophage production of tion of immune responses with nutritional treatments. Am. J. Clin. Nutr.
nitric oxide. J. Infect. Dis. 175, 1004 –1007. 66, 478S– 484S.
67. Chen, L-I., Pace, J. L., Russell, S. W., Morrison, D. C. (1996) Altered 91. Mazari, L., Lesourd, B. M. (1998) Nutrition influences on immune
regulation of inducible nitric oxide synthase expression in macrophages
responses in healthy aged persons. Mech. Ageing Dev. 104, 25– 40.
from senescent mice. Infect. Immun. 64, 4288 – 4298.
92. Barbul, A., Regan, M. C. (1995) Immune involvement in wound healing.
68. Beharka, A. A., Wu, D., Serafini, M., Meydani, S. N. (2002) Mechanism
Otolaryngol. Clin. North Am. 28, 955–968.
of vitamin E inhibition of cyclooxygenase activity in macrophages from
93. Swift, M. E., Burns, A. L., Gray, K. L., DiPietro, L. A. (2001) Age-related
old mice: role of peroxynitrite. Free Radic. Biol. Med. 32, 503–511.
69. Rollo, E. E., Denhardt, D. T. (1996) Differential effects of osteopontin on alterations in the inflammatory response to dermal injury. J. Invest.
the cytotoxic activity of macrophages from young and old mice. Immu- Dermatol. 117, 1027–1035.
nology 88, 642– 647. 94. Shallo, H., Plackett, T. P., Heinrich, S. A., Kovacs, E. J. (2003) Monocyte
70. Wu, D., Hayek, M. G., Meydani, S. (2001) Vitamin E and macrophage chemoattractant protein-1 (MCP-1) and macrophage infiltration into the
cyclooxygenase regulation in the aged. J. Nutr. 131, 382S–388S. skin after burn injury in aged mice. Burns 29, 641– 647.
71. Harizi, H., Juzan, M., Pitard, V., Moreau, J-F., Gualde, N. (2002) 95. Ashcroft, G. S., Horan, M. A., Ferguson, M. W. J. (1998) Aging alters the
Cycloxygenase-2-issued prostaglandin E2 enhances the production of inflammatory and endothelial cell adhesion molecule profiles during
endogenous IL-10, which down-regulates dendritic cell function. J. Im- human cutaneous wound healing. Lab. Invest. 78, 47–58.
munol. 168, 2255–2263. 96. Swift, M. E., Kleinman, H. K., DiPietro, L. A. (1999) Impaired wound
72. Harizi, H., Grosset, C., Gualde, N. (2003) Prostaglandin E2 modulates repair and delayed angiogenesis in aged mice. Lab. Invest. 79, 1479 –
dendritic cell function via EP2 and EP4 receptor subtypes. J. Leukoc. 1487.
Biol 73, 756 –763. 97. Vitale, M., Zamai, L., Neri, L. M., Galanzi, A., Facchini, A., Rana, R.,
73. Rieser, C., Papesh, C., Herold, M., Bock, G., Ramoner, R., Klocker, H., Cataldi, A., Papa, S. (1992) The impairment of natural killer function in
Bartsch, G., Thurnher, M. (1998) Differential deactivation of human the healthy aged is due to postbinding-deficient mechanism. Cell. Im-
dendritic cells by endotoxin desensitization: role of tumor necrosis fac- munol. 145, 1–10.
tor-␣ and prostaglandin E2. Blood 91, 3112–3117. 98. Ogata, K., Yokose, N., Tamura, H., An, E., Nakamura, K., Dan, K.,
74. Hilkens, C. M. U., Snijders, A., Snijdewint, F. G. M., Wierenga, E. A., Nomura, T. (1997) Natural killer cells in the late decades of human life.
Kapsenberg, M. L. (1996) Modulation of T-cell cytokine secretion by Clin. Immunol. Immunopathol. 84, 269 –275.
accessory cell-derived products. Eur. Respir. J. Suppl. 22, 90s–94s. 99. Mariani, E., Monaco, M. C. G., Cattini, L., Sinoppi, M., Facchini, A.
75. Woods, J. J., Grbic, J. T., Rodrick, M. L., Jordan, A., Mannick, J. A. (1994) Distribution and lytic activity of NK cell subsets in the elderly.
(1987) Suppression of interleukin-2 production in an animal model of Mech. Ageing Dev. 76, 177–187.
thermal injury is related to prostaglandin synthesis. Arch. Surg. 122, 100. Borrego, F., Alonso, M. C., Galiani, M. D., Carracedo, J., Ramirez, R.,
179 –184. Ostos, B., Pena, J., Solana, R. (1999) NK phenotypic markers and IL2
76. Plackett, T. P., Schilling, E. M., Faunce, D. E., Choudhry, M. A., Witte, response in NK cells from elderly people. Exp. Gerontol. 34, 253–265.
P. L., Kovacs, E. J. (2003) Aging enhances lymphocyte cytokine defects 101. McNerlan, S. E., Rea, I. M., Alexander, H. D., Morris, T. C. M. (1998)
after injury. FASEB J. 17, 688 – 689. Changes in natural killer cells, the CD57CD8 subset, and related cyto-
77. Adolfsson, O., Huber, B. T., Meydani, S. N. (2001) Vitamin E-enhanced kines in healthy aging. J. Clin. Immunol. 18, 31–38.
IL-2 production in old mice: naı̈ve but not memory T cells show in- 102. Mariani, E., Mariani, A. R., Meneghetti, A., Tarozzi, A., Cocco, L.,
creased cell division cycling and IL-2 producing capacity. J. Immunol. Facchini, A. (1998) Age-dependent decrease of NK cell phosphoinosi-
167, 3809 –3817. tide turnover during spontaneous but not Fc-mediated cytolytic activity.
78. Haynes, L., Linton, P. J., Eaton, S. M., Tonkonogy, S. L., Swain, S. L. Int. Immunol. 10, 981–989.
(1999) Interleukin 2, but not other common ␥ chain-binding cytokines, 103. Krishnaraj, R. (1997) Senescence and cytokines modulate the NK cell
can reverse the defect in generation of CD4 effector T cells from naı̈ve T expression. Mech. Ageing Dev. 96, 89 –101.
cells of aged mice. J. Exp. Med. 190, 1013–1024.
104. Dussault, I., Miller, S. C. (1994) Decline in natural killer cell-mediated
79. Mascarucci, P., Taub, D., Saccani, S., Paloma, M. A., Dawson, H., Roth,
immunosurveillance in aging mice–a consequence of reduced cell pro-
G. S., Ingram, D. K., Lane, M. A. (2001) Age-related changes in cytokine
duction and tumor binding capacity. Mech. Ageing Dev. 75, 115–129.
production by leukocytes in rhesus monkeys. Aging (Milano) 13, 85–94.
105. Kmiec, Z., Mysliwska, J., Rachon, D., Kotlarz, G., Sworczak, K., Mysliw-
80. Glaser, R., Maccallum, R. C., Laskowski, B. F., Malarkey, W. B.,
ski, A. (2001) Natural killer activity and thyroid hormone levels in young
Sheridan, J. F., Kiecott-Glaser, J. K. (2001) Evidence for a shift in the
Th1- and Th2-cytokine response associated with chronic stress and and elderly persons. Gerontology 47, 282–288.
aging. J. Gerontol. A Biol. Sci. Med. Sci. 56, M477–M482. 106. Mysliwska, J., Bryl, E., Zorena, K., Balon, J., Foerster, J., Mysliwski, A.
81. Renshaw, M., Rockwell, J., Engleman, C., Gewirtz, A., Katz, J., Samb- (1997) Overactivity of tumor necrosis factor-␣ but not interleukin 6 is
hara, S. (2002) Cutting edge: impaired Toll-like receptor expression and associated with low natural killer cytotoxic activity in the elderly. Ger-
function in aging. J. Immunol. 169, 4697– 4701. ontology 43, 158 –167.
82. Boehmer, E. D., Goral, J., Faunce, D. E., Kovacs, E. J. (2004) Age- 107. Mysliwska, J., Mysliski, A., Romanowski, P., Bigda, J., Sosnowska, D.,
dependent decrease in Toll-like receptor 4-mediated proinflammatory Foerster, J. (1992) Monocytes are responsible for depressed natural killer
cytokines by production and mitogen-activated protein kinase expres- (NK) activity in both young and elderly low NK responders. Gerontology
sion. J. Leukoc. Biol. 75, 342–349. 38, 41– 49.
83. Beharka, A. A., Meydani, M., Wu, D., Leka, L. S., Meydani, A., Meydani, 108. Ravaglia, G., Forti, P., Maioli, F., Bastagli, L., Facchini, A., Mariani, E.,
S. N. (2001) Interleukin-6 production does not increase with age. J. Savarino, L., Sassi, S., Cucinotta, D., Lenaz, G. (2000) Effects of micro-
Gerontol. A Biol. Sci. Med. Sci. 56, B81–B88. nutrient status on natural killer cell immune function in healthy free-
84. Bruunsgaard, H., Pedersen, B. K. (2003) Age-related inflammatory cy- living subjects aged ⱖ90 y. Am. J. Clin. Nutr. 71, 590 –598.
tokines and disease. Immunol. Allergy Clin. North Am. 23, 15–39. 109. De La Fuente, M., Miquel, J., Catalan, M. P., Victor, V. M., Guayerbas,
85. Kovacs, E. J., Grabowski, K. A., Duffner, L. A., Plackett, T. P., Gergory, N. (2002) The amount of thiolic antioxidant ingestion needed to improve
M. S. (2002) Survival and cell mediated immunity after burn injury in several immune functions is higher in aged than in adult mice. Free
aged mice. J. Amer. Aging Assoc. 25, 3–10. Radic. Res. 36, 119 –126.
298 Journal of Leukocyte Biology Volume 76, August 2004 http://www.jleukbio.org
110. Mikael, N., Mirza, N. M., Zaharian, B. I., Deulofeut, H., Salazar, M., 129. Dubey, D. P., Husain, Z., Levitan, E., Zurakowski, D., Mirza, N., Younes,
Yunis, E. J., Dubey, D. P. (1994) Genetic control of the decline of natural S., Coronell, C., Yunis, D., Yunis, E. J. (2000) The MHC influences NK
killer cell activity in aging mice. Growth Dev. Aging 58, 3–12. and NKT cell functions associated with immune abnormalities and
111. Hsueh, C-M., Chen, S-F., Ghanta, V. K., Hiramoto, R. N. (1996) In- lifespan. Mech. Ageing Dev. 113, 117–134.
volvement of cytokine gene expression in the age-dependent decline of 130. Inui, T., Nakagawa, R., Ohkura, S., Habu, Y., Koike, Y., Motoki, K.,
NK cell response. Cell. Immunol. 173, 221–229. Kuranaga, N., Fukasawa, M., Shinomiya, N., Seki, S. (2002) Age-asso-
112. Plett, A., Murasko, D. M. (2000) Genetic differences in the age-associ- ciated augmentation of the synthetic ligand-mediated function of mouse
ated decrease in inducibility of natural killer cells by interferon-␣/. NK1.1 Ag⫹ T cells: their cytokine production and hepatotoxicity in vivo
Mech. Ageing Dev. 112, 197–215. and in vitro. J. Immunol. 169, 6127– 6132.
113. Fernandes, G., Gupta, S. (1981) Natural killing and antibody-dependent
131. Leite-de-Moraes, M. C., Herbelin, A., Gouarin, C., Koezuka, Y., Schnei-
cytotoxicity by lymphocyte subpopulations in young and aging humans.
der, E., Dy, M. (2000) Fas/Fas ligand interactions promote activation-
J. Clin. Immunol. 1, 141–148.
114. Edwards, D. L., Avis, F. P. (1979) Antibody-dependent cellular cytotox- induced cell death of NK T lymphocytes. J. Immunol. 164, 4367– 4371.
icity effector cell capacity among normal individuals. J. Immunol. 123, 132. McGeer, P. L., Rogers, J., McGeer, E. G. (1994) Neuroimmune mecha-
1887–1893. nisms in Alzheimer disease pathogenesis. Alzheimer Dis. Assoc. Disord.
115. Edwards, B. K., Howe, H. L., Ries, L. A., Thun, M. J., Rosenberg, H. M., 8, 149 –158.
Yancik, R., Wingo, P. A., Jemal, A., Feigal, E. G. (2002) Annual report 133. Rogers, J., Schultz, J., Brachova, L., Lue, L. F., Webster, S., Bradt, B.,
to the nation on the status of cancer, 1973–1999, featuring implications Cooper, N. R., Moss, D. E. (1992) Complement activation and -amyloid-
of age and aging on U.S. cancer burden. Cancer 94, 2766 –2792. mediated neurotoxicity in Alzheimer’s disease. Res. Immunol. 143,
116. Jemal, A., Murray, T., Samuels, A., Ghafoor, A., Ward, E., Thun, M. J. 624 – 630.
(2003) Cancer statistics, 2003. CA Cancer J. Clin. 53, 5–26. 134. Lue, L. F., Rydel, R., Brigham, E. F., Yang, L. B., Hampel, H., Murphy
117. Albright, J. W., Albright, J. F. (1998) Impaired natural killer cell Jr., G. M., Brachova, L., Yan, S. D., Walker, D. G., Shen, Y., Rogers, J.
function as a consequence of aging. Exp. Gerontol. 33, 13–25. (2001) Inflammatory repertoire of Alzheimer’s disease and nondemented
118. Ogata, K., An, E., Shioi, Y., Nakamura, K., Luo, S., Yokose, N., Minami, elderly microglia in vitro. Glia 35, 72–79.
S., Dan, K. (2001) Association between natural killer cell activity and 135. Nagaki, K., Hiramatsu, S., Inai, S., Sasaki, A. (1980) The effect of aging
infection in immunologically normal elderly people. Clin. Exp. Immunol. on complement activity (CH50) and complement protein levels. J. Clin.
124, 392–397. Lab. Immunol. 3, 45–50.
119. Exley, M., Garcia, J., Balk, S. P., Porcelli, S. (1997) Requirements for 136. Oyeyink, G. O., Salimonu, L. S. (1999) Levels of complement compo-
CD1d recognition by human invariant V␣24⫹ CD4 –CD8 – T cells. J.
nents, immunoglobulins and acute phase proteins in plasma during aging
Exp. Med. 186, 109 –120.
in Nigeria. Afr. J. Med. Med. Sci. 28, 177–180.
120. Faunce, D. E., Gamelli, R. L., Choudhry, M. A., Kovacs, E. J. (2003) A
role for CD1d-restricted NKT cells in injury-associated T cell suppres- 137. Menzel, E. J., Zlabinger, G. J., Dunky, A., Steffen, C. (1988) Autoim-
sion. J. Leukoc. Biol. 73, 747–755. munity and T-cell subpopulations in old age. Arch. Gerontol. Geriatr. 7,
121. Gansert, J. L., Kiessler, V., Engele, M., Wittke, F., Rollinghoff, M., 249 –260.
Krensky, A. M., Porcelli, S. A., Modlin, R. L., Stenger, S. (2003) Human 138. Bellavia, D., Frada, G., Di Franco, P., Feo, S., Franceschi, C., Sansoni,
NKT cells express granulysin and exhibit antimycobacterial activity. P., Brai, M. (1999) C4, BF, C3 allele distribution and complement
J. Immunol. 170, 3154 –3161. activities in healthy aged people and Centenarians. J. Gerontol. A Biol.
122. Shao, H., Van Kaer, L., Sun, S. L., Kaplan, H. J., Sun, D. (2003) Sci. Med. Sci. 54, B150 –B153.
Infiltration of the inflamed eye by NKT cells in a rat model of experi- 139. Esparza-Gordillo, J., Soria, J. M., Buil, A., Souto, J. C., Almasy, L.,
mental autoimmune uveitis. J. Autoimmun. 21, 37– 45. Blangero, J., Fontcuberta, L., de Cordoba, S. R. (2003) Genetic deter-
123. Sonoda, K. H., Faunce, D. E., Taniguchi, M., Exley, M., Balk, S., minants of variation in the plasma levels of the C4-binding protein
Stein-Streilein, J. (2001) NK T cell-derived IL-10 is essential for the (C4BP) in Spanish families. Immunogenetics 54, 862– 866.
differentiation of antigen-specific T regulatory cells in systemic toler- 140. Ogata, R. T., Mathias, P., Bradt, B. M., Cooper, N. R. (1993) Murine
ance. J. Immunol. 166, 42–50. C4b-binding protein: mapping of the ligand binding site and the N-
124. Moore, K. W., O’Garra, A., de Waal Malefyt, R., Vieira, P., Mosmann, terminus of the pre-protein. J. Immunol. 150, 2273–2280.
T. R. (1993) Interleukin-10. Annu. Rev. Immunol. 11, 165–190. 141. Lavery, L. W., Goyns, M. H. (2002) Decline in the expression of C4
125. Dawson, H. D., Ross, A. C. (1999) Chronic marginal vitamin A status binding protein ␣-chain gene during ageing of the rat liver. Biogeron-
affects the distribution and function of T cells and natural T cells in aging
tology 3, 207–211.
Lewis rats. J. Nutr. 129, 1782–1790.
142. Cannon, J. G., Fiatarone, M. A., Fielding, R. A., Evans, W. J. (1994)
126. Miyaji, C., Wantanabe, H., Toma, H., Akisaka, M., Tomiyama, K., Sato,
Y., Abo, T. (2000) Functinal alterations of granulocytes, NK cells, and Aging and stress-induced changes in complement activation and neutro-
natural killer T cells in Centenarians. Hum. Immunol. 61, 908 –916. phil mobilization. J. Appl. Physiol. 76, 2616 –2620.
127. Tsukahara, A., Seki, S., Iiai, T., Moroda, T., Watanabe, H., Suzuki, S., 143. Kyrkanides, S., O’Banion, M. K., Whiteley, P. E., Daeschner, J. C.,
Tada, T., Hiraide, H., Hatakeyama, K., Abo, T. (1997) Mouse liver T Olschowka, J. A. (2001) Enhanced glial activation and expression of
cells: their change with aging and in comparison with peripheral T cells. specific CNS inflammation-related molecules in aged versus young rats
Hepatology 26, 301–309. following cortical stab injury. J. Neuroimmunol. 119, 269 –277.
128. Poynter, M. E., Mu, H-H., Chen, X-P., Daynes, R. A. (1997) Activation 144. Hazlett, L. D., Masinick-McClellan, S. A., Barrett, R. P. (1999) Com-
of NK1.1⫹ T cells in vitro and their possible role in age-associated plement defects in aged mice compromise phagocytosis of Pseudomonas
changes in inducible IL-4 production. Cell. Immunol. 179, 22–29. aeruginosa. Curr. Eye Res. 19, 26 –32.
Plackett et al. Innate immunity in aging 299
You might also like
- USMLE Road Map ImmunologyDocument242 pagesUSMLE Road Map ImmunologyFernanda Granillo100% (9)
- The Morphology of Human Blood Cells - IllustrationDocument86 pagesThe Morphology of Human Blood Cells - Illustrationalphaceta100% (3)
- Fulop 2016Document8 pagesFulop 2016GembongSatriaMahardhikaNo ratings yet
- The FEBS Journal - 2021 - Zhang - Hallmarks of The Aging T Cell SystemDocument20 pagesThe FEBS Journal - 2021 - Zhang - Hallmarks of The Aging T Cell SystemSubhajit DuttaNo ratings yet
- Canan 2014 JLBDocument1 pageCanan 2014 JLBNandan GokhaleNo ratings yet
- Annals of The New York Academy of Sciences - 2009 - Bauer - The Role of Stress Factors During Aging of The Immune SystemDocument14 pagesAnnals of The New York Academy of Sciences - 2009 - Bauer - The Role of Stress Factors During Aging of The Immune SystemegrdfgNo ratings yet
- Aging and The Immune System: The Impact of Immunosenescence On Viral Infection, Immunity and Vaccine ImmunogenicityDocument18 pagesAging and The Immune System: The Impact of Immunosenescence On Viral Infection, Immunity and Vaccine Immunogenicityskyyblue10No ratings yet
- Microparticulas ArdsDocument18 pagesMicroparticulas ArdsARELHI ROSARIO GARCIA MONROYNo ratings yet
- FMD Nature Paper s41467-024-45260-9Document13 pagesFMD Nature Paper s41467-024-45260-9davidbearx3No ratings yet
- Microscopy Res Technique - 2001 - Southwood - Molecular Pathways of Oligodendrocyte Apoptosis Revealed by Mutations inDocument9 pagesMicroscopy Res Technique - 2001 - Southwood - Molecular Pathways of Oligodendrocyte Apoptosis Revealed by Mutations inAnglia LopesNo ratings yet
- Inflammatory Networks in The Control of Spermatogenesis: Chronic Inflammation in An Immunologically Privileged Tissue?Document23 pagesInflammatory Networks in The Control of Spermatogenesis: Chronic Inflammation in An Immunologically Privileged Tissue?Bobby's SetyabudiNo ratings yet
- Science Abe4832 FullDocument20 pagesScience Abe4832 FullsussanNo ratings yet
- Neutrophils Induce Paracrine Telomere Dysfunction and Senescence in ROS-dependent MannerDocument19 pagesNeutrophils Induce Paracrine Telomere Dysfunction and Senescence in ROS-dependent MannerValen EstevezNo ratings yet
- Aging V15i13 204896Document24 pagesAging V15i13 204896Zewdie Tadesse GebremariamNo ratings yet
- Influence of AgingDocument16 pagesInfluence of AgingSCIH HFCPNo ratings yet
- Review Article: Oral Lichen Planus As A Preneoplastic Inflammatory ModelDocument9 pagesReview Article: Oral Lichen Planus As A Preneoplastic Inflammatory ModelFakhrur RaziNo ratings yet
- Final Hoo Gen MsDocument21 pagesFinal Hoo Gen MsbadrusharryNo ratings yet
- Ra and Cardiovascular MorbidityDocument9 pagesRa and Cardiovascular MorbidityKeerthi SagarNo ratings yet
- Sirs CarsDocument14 pagesSirs CarsJosé ChalcoNo ratings yet
- Immuno Senescence PublishedDocument5 pagesImmuno Senescence PublishedAcengHamudinNo ratings yet
- Biomarcadores en InmunosupresionDocument10 pagesBiomarcadores en InmunosupresionRicardo GarciaNo ratings yet
- Chapter 15 - The Aging Immune System Dysregulation, Compensatory Mechanisms, and Prospects For Intervention PDFDocument25 pagesChapter 15 - The Aging Immune System Dysregulation, Compensatory Mechanisms, and Prospects For Intervention PDFCayadi Sidarta Antonius PocipNo ratings yet
- Dr. Dwi Ngestiningsih - Penurunan Imunitas Lansia+LOGO BARUDocument27 pagesDr. Dwi Ngestiningsih - Penurunan Imunitas Lansia+LOGO BARUMuhammad Zul Fahmi AkbarNo ratings yet
- Moos 2020 Klotho Pathways Myelination DisordeDocument12 pagesMoos 2020 Klotho Pathways Myelination DisordeFiterman AdrianNo ratings yet
- Mechanisms of Ageing and Development: SciencedirectDocument8 pagesMechanisms of Ageing and Development: SciencedirectAgustin LopezNo ratings yet
- Cellular Aging and CancerDocument12 pagesCellular Aging and CancerVempati Rahul KumarNo ratings yet
- Bqab 136Document12 pagesBqab 136Tatta CamposNo ratings yet
- 2019 # Ribeiro Regulation of Innate Immune Responses by PlateletsDocument9 pages2019 # Ribeiro Regulation of Innate Immune Responses by PlateletsMaria Eduarda AquinoNo ratings yet
- Immunosenescence, Inflammaging and Cell SenescenceDocument63 pagesImmunosenescence, Inflammaging and Cell SenescenceArthur YanezNo ratings yet
- Hiv No VelhoDocument20 pagesHiv No VelhoSCIH HFCPNo ratings yet
- The Interplay Between Immunosenescence and Age-Related DiseasesDocument14 pagesThe Interplay Between Immunosenescence and Age-Related Diseasesadibashafique_214280No ratings yet
- Cellular Immunology: Lorena Álvarez-Rodríguez, Marcos López-Hoyos, Pedro Muñoz-Cacho, Víctor Manuel Martínez-TaboadaDocument9 pagesCellular Immunology: Lorena Álvarez-Rodríguez, Marcos López-Hoyos, Pedro Muñoz-Cacho, Víctor Manuel Martínez-TaboadaFernanda Camargo NunesNo ratings yet
- Epigenetic Clocks Aging and CancerDocument3 pagesEpigenetic Clocks Aging and Cancerzhe zhNo ratings yet
- 432 440 PDFDocument9 pages432 440 PDFjamesNo ratings yet
- Evidence and Perspectives of Cell Senescence in Neurodegenerative DiseasesDocument11 pagesEvidence and Perspectives of Cell Senescence in Neurodegenerative DiseasesKarel GuevaraNo ratings yet
- Aging, Cancer, and The Mechanisms Behind This Relationship That Continue To Evade DetectionDocument13 pagesAging, Cancer, and The Mechanisms Behind This Relationship That Continue To Evade Detectionketpoc22No ratings yet
- Epigenetics and Aging: ReviewDocument20 pagesEpigenetics and Aging: ReviewDeepak PanwarNo ratings yet
- Review Sepsis CCM 2007Document9 pagesReview Sepsis CCM 2007Nicole MariaNo ratings yet
- From Endoplasmic-Reticulum Stress To The Inflammatory ResponseDocument22 pagesFrom Endoplasmic-Reticulum Stress To The Inflammatory ResponseivanovichNo ratings yet
- Depressive SintomysDocument6 pagesDepressive SintomysBráulio LimaNo ratings yet
- The Cell Biology of Aging: Race Diloreto and Coleen T. MurphyDocument8 pagesThe Cell Biology of Aging: Race Diloreto and Coleen T. MurphyLeoberto Batista Pereira SobrinhoNo ratings yet
- Immunology of Idiopathic Nephrotic Syndrome: ReviewDocument12 pagesImmunology of Idiopathic Nephrotic Syndrome: ReviewDung TranNo ratings yet
- Cellular Immune Responses in The Pathophysiology of 2021Document24 pagesCellular Immune Responses in The Pathophysiology of 2021Zakkiyatus ZainiyahNo ratings yet
- EditorialDocument2 pagesEditorialsebastoto778No ratings yet
- Inflammatory Monocytes Suppression of Vaccine Immunity By: References Cites 51 ArticlesDocument11 pagesInflammatory Monocytes Suppression of Vaccine Immunity By: References Cites 51 Articlesthh uuNo ratings yet
- Lung Involvement in Childhood Measles: Severe Immune Dysfunction Revealed by Quantitative ImmunohistochemistryDocument10 pagesLung Involvement in Childhood Measles: Severe Immune Dysfunction Revealed by Quantitative Immunohistochemistryapi-286128974No ratings yet
- Galley - Inflammation and ImmunityDocument119 pagesGalley - Inflammation and Immunityla_herbolariaNo ratings yet
- Baker Petersen 2018 Cellular Senescence in Brain Aging and Neurodegenerative Diseases - RAFAELA FAUSTINO LACERDA de SOUZADocument10 pagesBaker Petersen 2018 Cellular Senescence in Brain Aging and Neurodegenerative Diseases - RAFAELA FAUSTINO LACERDA de SOUZAraquel rodriguesNo ratings yet
- Immunological Changes With Age and Innovative Approaches To Bolster Immune Function in Older AdultsDocument11 pagesImmunological Changes With Age and Innovative Approaches To Bolster Immune Function in Older AdultsKIU PUBLICATION AND EXTENSIONNo ratings yet
- AMPK Function in Aging Process: Rocío Ruiz, Eva María Pérez-Villegas and Ángel Manuel CarriónDocument11 pagesAMPK Function in Aging Process: Rocío Ruiz, Eva María Pérez-Villegas and Ángel Manuel CarriónNadilla NatashaNo ratings yet
- Molecular and Genetic Aspects of AgingDocument6 pagesMolecular and Genetic Aspects of AgingRahul surya BobbiliNo ratings yet
- Aging and Stem CellsDocument4 pagesAging and Stem Cellsdanielc503No ratings yet
- Sle 3Document8 pagesSle 3Dianu GutiérrezNo ratings yet
- Endothelial Barrier Dysfunction in Septic Shock: ReviewDocument17 pagesEndothelial Barrier Dysfunction in Septic Shock: ReviewSola SacraNo ratings yet
- Editorial: DNA Damage: Health and LongevityDocument2 pagesEditorial: DNA Damage: Health and LongevityAngelica CobosNo ratings yet
- Brain Aging and Regeneration After InjuriesDocument16 pagesBrain Aging and Regeneration After InjuriesProsen21No ratings yet
- The Biology of Aging: The Current Research AgendaDocument10 pagesThe Biology of Aging: The Current Research Agendagriffone1No ratings yet
- Ciclo Celular y CáncerDocument12 pagesCiclo Celular y CáncerAlfonso De la FuenteNo ratings yet
- (Jean Langhorne (Editor) ) Immunology and ImmunopatDocument239 pages(Jean Langhorne (Editor) ) Immunology and Immunopatclaudia lilianaNo ratings yet
- Chronic Inflammation (Inflammaging) and Its Potential Contribution To Age-Associated DiseasesDocument6 pagesChronic Inflammation (Inflammaging) and Its Potential Contribution To Age-Associated DiseasesReindeer MerkaatsNo ratings yet
- Cell Death in Health and DiseaseDocument11 pagesCell Death in Health and DiseaseSurya DinataNo ratings yet
- Clinical Genetics and Genomics of AgingFrom EverandClinical Genetics and Genomics of AgingJuan Carlos Gomez-VerjanNo ratings yet
- cMyC MIDocument3 pagescMyC MISNo ratings yet
- TyG ATSDocument10 pagesTyG ATSSNo ratings yet
- TG HDL ObeseDocument7 pagesTG HDL ObeseSNo ratings yet
- TyG preDMDocument12 pagesTyG preDMSNo ratings yet
- TyG VascularagingDocument8 pagesTyG VascularagingSNo ratings yet
- Warts, Herpes Simplex, and Other Viral InfectionsDocument37 pagesWarts, Herpes Simplex, and Other Viral InfectionsSNo ratings yet
- Bacterial InfectionsDocument47 pagesBacterial InfectionsSNo ratings yet
- Bromothymol BlueDocument6 pagesBromothymol BlueSNo ratings yet
- Principles of Drug TherapyDocument8 pagesPrinciples of Drug TherapySNo ratings yet
- Pulmonary EmbolismDocument17 pagesPulmonary EmbolismSNo ratings yet
- Alpha Ketoglutaric AcidDocument9 pagesAlpha Ketoglutaric AcidSNo ratings yet
- Palmitoyl-D-carnitine (Chloride)Document7 pagesPalmitoyl-D-carnitine (Chloride)SNo ratings yet
- Product SpecificationDocument1 pageProduct SpecificationSNo ratings yet
- Bromophenol BlueDocument20 pagesBromophenol BlueSNo ratings yet
- Cesium ChlorideDocument7 pagesCesium ChlorideSNo ratings yet
- AlbuminaDocument10 pagesAlbuminaSNo ratings yet
- Ceruleum BromfenolDocument5 pagesCeruleum BromfenolSNo ratings yet
- Fast Blue BDocument9 pagesFast Blue BSNo ratings yet
- Cerium Chloride HeptahydrateDocument11 pagesCerium Chloride HeptahydrateSNo ratings yet
- Acid Tungsto FosforicDocument7 pagesAcid Tungsto FosforicSNo ratings yet
- CatalaseDocument6 pagesCatalaseSNo ratings yet
- Safety Data Sheet: DexamethasoneDocument9 pagesSafety Data Sheet: Dexamethasonehadeer YoussriNo ratings yet
- 1-Naphthyl Phosphate MonosodiumDocument7 pages1-Naphthyl Phosphate MonosodiumSNo ratings yet
- AlanineDocument10 pagesAlanineSNo ratings yet
- 3 (3,4 Dihydroxyphenyl) AlaninDocument5 pages3 (3,4 Dihydroxyphenyl) AlaninSNo ratings yet
- Safety Data Sheet: Tci Europe N.VDocument5 pagesSafety Data Sheet: Tci Europe N.VSNo ratings yet
- Acute Renal FailureDocument6 pagesAcute Renal FailureSNo ratings yet
- Acoustic NeuromaDocument2 pagesAcoustic NeuromaSNo ratings yet
- Acute Respiratory Distress SyndromeDocument2 pagesAcute Respiratory Distress SyndromeSNo ratings yet
- Acne VulgarisDocument2 pagesAcne VulgarisSNo ratings yet
- Curcuma 2020 Fcell-08-00479Document10 pagesCurcuma 2020 Fcell-08-00479Jorge Luis Plasencia CubaNo ratings yet
- Hema Lec CompiledDocument68 pagesHema Lec Compiledchris andrieNo ratings yet
- Pathology Important Solved BCQs 5th Semester MBBS + 3rd Semester BDS LUMHSDocument57 pagesPathology Important Solved BCQs 5th Semester MBBS + 3rd Semester BDS LUMHSNaheed Jatoi100% (1)
- Wound Healing in The Oral Mucosa: Patricio C. Smith and Constanza MartínezDocument14 pagesWound Healing in The Oral Mucosa: Patricio C. Smith and Constanza MartínezNadira NurinNo ratings yet
- Acute Myelogenous Leukemia - Patho, Anatomy, and Diagnostic TestDocument66 pagesAcute Myelogenous Leukemia - Patho, Anatomy, and Diagnostic TestCharles Kevin L. Bataga33% (3)
- Overview of The Immune System 2020Document31 pagesOverview of The Immune System 2020mehakNo ratings yet
- Anti Inflammatory Prospective Study of Commiphora Mukul (Guggul) On Wistar Albino RatsDocument54 pagesAnti Inflammatory Prospective Study of Commiphora Mukul (Guggul) On Wistar Albino RatsKrish NalimelaNo ratings yet
- Acute and Chronic InflammationDocument52 pagesAcute and Chronic Inflammationjames20123100% (1)
- The Role of Inflammation and Genetics in Periodontal DiseaseDocument14 pagesThe Role of Inflammation and Genetics in Periodontal DiseaseNishtha KumarNo ratings yet
- Red Blood Cell BiochemistryDocument19 pagesRed Blood Cell BiochemistryPrincewill SeiyefaNo ratings yet
- GoutDocument12 pagesGoutEarle Jimenez Niervo RN100% (1)
- White Paper Parameters and Species Exigo h400 - Wpe - 34071 1 PDFDocument12 pagesWhite Paper Parameters and Species Exigo h400 - Wpe - 34071 1 PDFОльга ДовженкоNo ratings yet
- Hematology 2009Document820 pagesHematology 2009Isak Isak IsakNo ratings yet
- Biology 12 - Chapter 11 - Blood - Chapter Notes: Here Is A Micrographs Showing Formed Elements in Human BloodDocument7 pagesBiology 12 - Chapter 11 - Blood - Chapter Notes: Here Is A Micrographs Showing Formed Elements in Human BloodChan KarlokNo ratings yet
- Peripheral Blood LeukocytesDocument21 pagesPeripheral Blood LeukocytesValentina SuescunNo ratings yet
- ICSH Recommendations For The Standardization of Peripheral Blood Cell Morphological Features PDFDocument17 pagesICSH Recommendations For The Standardization of Peripheral Blood Cell Morphological Features PDFSaraswati Wulandari HartonoNo ratings yet
- 10 - Chapter - 4 PDFDocument163 pages10 - Chapter - 4 PDFBalakrishna GopinathNo ratings yet
- Role of Sugars in Human Neutrophilic PhagocytosisDocument5 pagesRole of Sugars in Human Neutrophilic Phagocytosisdumitrudragos100% (2)
- Pathology Week 2 p1-18Document18 pagesPathology Week 2 p1-18zeroun24100% (1)
- Pact Immunocompromised Patients 2010 PDFDocument67 pagesPact Immunocompromised Patients 2010 PDFBreno PontesNo ratings yet
- Biomedical Research Associate Scientist in Houston TX Resume Sandhya DasDocument6 pagesBiomedical Research Associate Scientist in Houston TX Resume Sandhya DasSandhyaDas2100% (1)
- BUUN Immunomodulatory Effects of Massage in Skeletal MuscleDocument133 pagesBUUN Immunomodulatory Effects of Massage in Skeletal Muscledavidescu5costinNo ratings yet
- HBII Lab Manual 14-1-2019Document50 pagesHBII Lab Manual 14-1-2019Ahmed Khaled Abo EldahabNo ratings yet
- Periodontology Bartolucci OneDocument334 pagesPeriodontology Bartolucci Onekevinwu003100% (10)
- Ch2 InflamDocument49 pagesCh2 InflamleartaNo ratings yet
- Surgery A 1.1Document4 pagesSurgery A 1.1Aisha Al JenaibiNo ratings yet
- Blood Anatomy and Physiology ReviewDocument20 pagesBlood Anatomy and Physiology ReviewStacey CamilleNo ratings yet
- Neutrophils in DiseaseDocument16 pagesNeutrophils in DiseaseAbhishaik SinghNo ratings yet