Professional Documents
Culture Documents
CH7 Sykes Electrophilic-Nucleophilic Addition
Uploaded by
Tazyinul Qoriah AlfauziahOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
CH7 Sykes Electrophilic-Nucleophilic Addition
Uploaded by
Tazyinul Qoriah AlfauziahCopyright:
Available Formats
CH7 Sykes Electrophilic-Nucleophilic Addition.
docx
2012Oct25
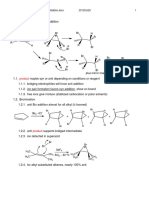
1. Mechanism of electrophilic addition
1.1. product maybe syn or anti depending on conditions or reagent 1.1.1. bridging electrophiles will force anti addition 1.1.2. ion pair formation favors syn addition, show on board 1.1.3. free ions give mixture (stablized carbocation or polar solvents) 1.2. Bromination 1.2.1. anti Br2 addition almost for all alkyl (I2 favored)
1.2.2. anti product supports bridged intermediate 1.2.3. ion detected in superacid
1.2.4. for alkyl substituted alkenes, nearly 100% anti
CH7 Sykes Electrophilic-Nucleophilic Addition.docx
2012Oct25
1.2.5. less stereospecific for aromatic substitutents (favored)
1.2.6. less stereospecific in polar solvents (favored)
1.3. substituent effects - electron donation delocalization accelates
1.4. Chlorination 1.4.1. similar to bromination but bridged species less stable 1.4.2. more syn formation 1.5. Fluorination to violent 1.6. Iodination - product unstable 1.7. Mixed halogenations: BrCl > BrBr > ICl > IBr > I-I 1.7.1. Least electronegative atom adds 1.7.2. Faster for stronger CX bond and more electronegative leaving group 2. HX addition: Markovnikov's rule: what is it? 2.1. HI, HBr, HCl, H2SO4, H2O, ROH, RSH, RCOOH all add to alkenes in acid conditions 2.2. H+ does not bridge 2.3. AdE2 bimolecular stepwise addition: RDS is H+ addition
2.4. Markovnikov addition is preferred- proton adds to least substuted carbon (form most stable cation
CH7 Sykes Electrophilic-Nucleophilic Addition.docx
2012Oct25
2.5. Carbon cation rearrangements can occur
2.6. HX adds H+ in non-polar solvents 2.7. Protonated solvent adds in protic solvent, i.e. H3O+ 2.8. HI > HBr > HCl >> HF 2.9. Concerted addition, AdE3, termolecular concerted addition, reverse of E2 2.9.1. break bond, make 2 bonds 2.9.2. addition is anti 2.9.3. mechanism favored when solvent participates (HBr in HOAc)
2.10. Stepwise addition(AdE2) is favored by strong acids (CF3SO3H in HOAc) 3. Hydration 3.1. Acid catalyzed
CH7 Sykes Electrophilic-Nucleophilic Addition.docx
2012Oct25
3.2. Reverse of dehydration of alcohols 3.3. Need strong acid with poor nucleophile/conjugate base,HBr/water? 3.4. Dilute sulfuric acid common, concentrated sulfuric acid 4. Hydroboration-oxidation convert alkene to alcohol (water addition hydration) 4.1. Hydroborane equilibrium with diborane 4.1.1. BH3 has empty orbital and BH bond is polar (H more electronegative) 4.1.2. B accepts electrons! BH3 is a Lewis acid and hydride donor? 4.1.3. Only 4 electrons total in two bridge, low barrier for dissociation
4.2. Borane addition to C=C 4.2.1. Boron accepts electrons 4.2.2. attacheds to least substituted carbon (Markovnikov) 4.2.3. H migration to electron deficient center 4.2.4. often concerted- no C-C rotation, stereochemistry is preserved
4.2.5. Two more equivalents of hydride: trialkylborane
4.3. Hydro oxidation- transfer to oxygen 4.3.1. Trialkylborane, equivalent to carbon cation without charge 4.3.2. Hydroperoxide anion very good nucleophile, adds to electron deficient center
CH7 Sykes Electrophilic-Nucleophilic Addition.docx
2012Oct25
4.3.3. R migration to electron deficient O center
4.4. Base hydrolysis 4.4.1. Hydroxide adds to alkyl borate 4.4.2. Form tetrahedral intermediate from trigonal intermediate common process 4.4.3. substitution of OR by OH, addition-elimination, not an SN2 4.4.4. three equivalent of alcohols
4.5. why not just do regular water addition? 4.5.1. Avoid rearrangement of cations 4.5.2. Stereospecific retain stereochemistry during addition and migrations 4.5.3. Complementary stereochemistry to acid catalyzed hydration- anti-Markovnikov
4.5.4. H. C. Brown 5. Carbon cation addition to alkene 5.1.1. Dimer/trimer/polymerization 5.1.2. Potonate electrons/cation adds to electrons
5.1.3. Further addition can occur but steric inhibition slowes down reaction 5.1.4. Dissociation of proton forms new alkene
5.1.5. Hydrogenation yields isooctane: 100 % octane rating
CH7 Sykes Electrophilic-Nucleophilic Addition.docx
2012Oct25
6. Oxidative hydroxylation- skip Osmium tetroxide and permanganate 6.1. Peracid is self activating, electron deficient center 6.2. Oxygen adds to both carbons simultaneously, form epoxide 6.3. No rotation about CC bond, preserve stereochemistry 6.4. Similar chemistry to bromonium but epoxide is stable product
6.5. Acid or base catalyzed hydrolysis yields diol: anti addition
6.6. Asymmetric epoxides have different products for acid and base catalyzed reactions 6.6.1. Base conditions favor nucleophile attack least substituted carbon (question)
6.6.2. Acid conditions create positive charge at more substituted carbon
7. Skip Hydrogenation and Ozonolysis (later in cycloaddition) 8. Addition to dienes
CH7 Sykes Electrophilic-Nucleophilic Addition.docx
2012Oct25
8.1. 1,2 versus 1,4 addition (aprotic v. protic)
8.2. HCl addition 8.2.1. at 60 oC 75% 1,2 addition, 20-25% 1,4 addition 8.2.2. 20 oC? 25% 1,2 addition, 75% 1,4 addition 8.2.3. Ion pairing keeps Cl from roaming and 1,2 is favored 8.2.4. Higher temperature 1,4 is favored 8.2.5. Heating pure 1,2 or 1,4 get the same mixture at high temperature, why?
9. Skip Diels Alder (chapter 12) 10. Nucleophilic addition 10.1. Electron withdrawing group stabilze negative charge
10.2. Michael reaction with carbon nulceophiles 10.2.1. 1,2 addition favored for more reactive group without steric hindrance 10.2.2. 1,4 addition favored for strerically unhindered 4 position if 2 position is blocked?
10.3. Enolates prefer conjugate addition (less ion pairing)
CH7 Sykes Electrophilic-Nucleophilic Addition.docx
2012Oct25
You might also like
- Practice Makes Perfect in Chemistry: Acids, Bases, and Salts with AnswersFrom EverandPractice Makes Perfect in Chemistry: Acids, Bases, and Salts with AnswersNo ratings yet
- Mechanisms of Electrophilic Addition ReactionsDocument8 pagesMechanisms of Electrophilic Addition ReactionsPutrianty AnnisaNo ratings yet
- (Lecture 3) Carbonyls and AminesDocument34 pages(Lecture 3) Carbonyls and AminesKasraSrNo ratings yet
- FullDocument5 pagesFullElmer Jhon CasNo ratings yet
- Aldehydes, Ketones and Carboxylic AcidsDocument9 pagesAldehydes, Ketones and Carboxylic AcidsAnand RawatNo ratings yet
- Organic ReactionsDocument44 pagesOrganic ReactionsJanhavi PatilNo ratings yet
- Aldehydes and Ketones IIDocument46 pagesAldehydes and Ketones IImidohemaNo ratings yet
- REVIEWER - Alkoxide To OxidationDocument18 pagesREVIEWER - Alkoxide To OxidationKeren Keziah TangarorangNo ratings yet
- Organic Chemistry Chapter 8Document41 pagesOrganic Chemistry Chapter 8채종희No ratings yet
- Organic Chemistry Alkynes ReactionsDocument9 pagesOrganic Chemistry Alkynes ReactionsAnthony KwofieNo ratings yet
- Alkenes: Reactions and MechanismsDocument45 pagesAlkenes: Reactions and MechanismsBritney PattersonNo ratings yet
- Reactions of Aldehydes and KetonesDocument12 pagesReactions of Aldehydes and KetonespramodpsmpharmNo ratings yet
- Alcohol Phenol and Ether PYQ Solution - 18290254 - 2023 - 06 - 20 - 12 - 09Document3 pagesAlcohol Phenol and Ether PYQ Solution - 18290254 - 2023 - 06 - 20 - 12 - 09telate6613No ratings yet
- Synthetic of CyclohexanoneDocument15 pagesSynthetic of CyclohexanoneRadiatul Awalia AmirNo ratings yet
- Aldehydes & Ketones: Sorsogon State CollegeDocument12 pagesAldehydes & Ketones: Sorsogon State CollegeElizabeth BáthoryNo ratings yet
- Electrophile: Organic ChemistryDocument7 pagesElectrophile: Organic ChemistryJesse SawianNo ratings yet
- Addition ReactionDocument34 pagesAddition Reactionmichot feleguNo ratings yet
- 5294 H+i Assignment No 2 M WaseemDocument6 pages5294 H+i Assignment No 2 M WaseemM WaseemNo ratings yet
- Pre Lab QuestionsDocument5 pagesPre Lab QuestionsMatthew VillanuevaNo ratings yet
- Summary of Organic ReactionsDocument21 pagesSummary of Organic ReactionsMarie St. LouisNo ratings yet
- Hydrocarbon Chemistry CHE 225 Reactions of AlkynesDocument7 pagesHydrocarbon Chemistry CHE 225 Reactions of AlkynesNurul 'Uyun AhmadNo ratings yet
- Esters Properties - ChemicalDocument7 pagesEsters Properties - ChemicalEshaal KhanNo ratings yet
- Chapter 11 - The Chemistry of Ethers, Epoxides, Glycols, and SulfidesDocument7 pagesChapter 11 - The Chemistry of Ethers, Epoxides, Glycols, and SulfidesJohn SmithNo ratings yet
- 4.2 Hydration of AlkenesDocument33 pages4.2 Hydration of AlkenesDawit BirhanuNo ratings yet
- Chap 8 LDocument127 pagesChap 8 Lxp4gb45jjqNo ratings yet
- Ethers and Epoxides: Ethers Nomenclature of EthersDocument10 pagesEthers and Epoxides: Ethers Nomenclature of EtherssarahNo ratings yet
- CHEM111 Alcohol, Phenol, EtherDocument26 pagesCHEM111 Alcohol, Phenol, EtherAgatha joy MadrazoNo ratings yet
- Common Mechanisms in Biological ChemistryDocument42 pagesCommon Mechanisms in Biological Chemistrygyogi1989No ratings yet
- Reduction Agents Organic ChemistryDocument55 pagesReduction Agents Organic ChemistryvgvijuNo ratings yet
- Organic Chemistry II Chapter 22 Notes on Alpha Substitution ReactionsDocument9 pagesOrganic Chemistry II Chapter 22 Notes on Alpha Substitution ReactionsKuandi TanNo ratings yet
- Organic Reactions and MechanismDocument51 pagesOrganic Reactions and MechanismAbhay Kumar Nayak75% (8)
- Free Radical Substitution and Electrophilic AdditionDocument17 pagesFree Radical Substitution and Electrophilic Additionchicko33No ratings yet
- CHE1502/202/2/2017 Tutorial Letter Provides Important InformationDocument8 pagesCHE1502/202/2/2017 Tutorial Letter Provides Important InformationLeigh MakanNo ratings yet
- Reaction of Aldehydes & Ketones-2Document30 pagesReaction of Aldehydes & Ketones-2MGoyalNo ratings yet
- Kondensasi Karbonil FarDocument71 pagesKondensasi Karbonil FarAzra Al AmanahNo ratings yet
- Module 4 Aldehydes and KetonesDocument11 pagesModule 4 Aldehydes and Ketonesaliya margo gonzalesNo ratings yet
- Chapter 19. Aldehydes and KetonesDocument63 pagesChapter 19. Aldehydes and Ketonesxzgnrmqt9nNo ratings yet
- Electrophilic Addition of Alkenes NotesDocument17 pagesElectrophilic Addition of Alkenes NotesAnanda Vijayasarathy100% (1)
- Reactions With BenzeneDocument25 pagesReactions With BenzeneVicky75% (4)
- Chapter 4 AlkenesDocument40 pagesChapter 4 Alkenesdead soulNo ratings yet
- 1515565547CHE P1 M25 EtextDocument17 pages1515565547CHE P1 M25 Etextafrozshams1869No ratings yet
- ArenesDocument5 pagesArenes林琪No ratings yet
- Chap 7Document35 pagesChap 7أسامة المنتصرNo ratings yet
- Chapter-12 - Aldehydes-Ketones-and-Carboxylic-Acids Important QuestionDocument13 pagesChapter-12 - Aldehydes-Ketones-and-Carboxylic-Acids Important QuestionPonuNo ratings yet
- Aldehyde KetoneDocument25 pagesAldehyde KetoneIpshita PathakNo ratings yet
- Addition Reactions of Alkenes: Hydrogenation, Hydration, Halogenation, OzonolysisDocument89 pagesAddition Reactions of Alkenes: Hydrogenation, Hydration, Halogenation, OzonolysisasyaanazNo ratings yet
- BP401T-5Document18 pagesBP401T-5saheedvkNo ratings yet
- Chapter 8 Alkenes and Alkynes II4Document7 pagesChapter 8 Alkenes and Alkynes II4wimal nayanaNo ratings yet
- Chapter 10 Haloalkanes and HaloarenesDocument19 pagesChapter 10 Haloalkanes and HaloarenesSujithNo ratings yet
- Benzene Ring Structure and ReactionsDocument8 pagesBenzene Ring Structure and ReactionsRichard LaneNo ratings yet
- Acids and Bases, Part 1: Acid/Base Speciation and Exact Solutions To Acid/Base ProblemsDocument76 pagesAcids and Bases, Part 1: Acid/Base Speciation and Exact Solutions To Acid/Base ProblemswastequestNo ratings yet
- Hydration of AlkynesDocument8 pagesHydration of AlkynesAnthony BasantaNo ratings yet
- Addition Reactions at sp2 CarbonsDocument85 pagesAddition Reactions at sp2 CarbonsPrarabdha SharmaNo ratings yet
- Organic Chemistry Study SheetDocument22 pagesOrganic Chemistry Study SheetJosephine Chen100% (1)
- Lecture 6 WordsDocument5 pagesLecture 6 WordsDonald LetsoNo ratings yet
- Carberylic Acid.Document22 pagesCarberylic Acid.afrNo ratings yet
- Palladium Reagents and Catalysts: New Perspectives for the 21st CenturyFrom EverandPalladium Reagents and Catalysts: New Perspectives for the 21st CenturyNo ratings yet
- Title Using Time New Roman 16 Bold, Should Be Simple, and Represent The Article ContentDocument2 pagesTitle Using Time New Roman 16 Bold, Should Be Simple, and Represent The Article ContentTazyinul Qoriah AlfauziahNo ratings yet
- Antifungal Activity of Eugenol Against Botrytis Cinerea: Chunmei Wang, Jie Zhang, Hao Chen, Yongjian FanDocument7 pagesAntifungal Activity of Eugenol Against Botrytis Cinerea: Chunmei Wang, Jie Zhang, Hao Chen, Yongjian FanTazyinul Qoriah AlfauziahNo ratings yet
- Determination of Aspirin by Indirect TitrationDocument3 pagesDetermination of Aspirin by Indirect TitrationRica Marquez100% (2)
- 12 Principles of Green Chemistry: PreventionDocument2 pages12 Principles of Green Chemistry: PreventionAnonymous 2ABu0iNo ratings yet
- FORMULAASPIRIN TABLETDocument2 pagesFORMULAASPIRIN TABLETTazyinul Qoriah AlfauziahNo ratings yet
- Adverse Drug Reactions of Imatinib in Patients With Chronic Myeloid Leukemia-A Single-Center Surveillance StudyDocument4 pagesAdverse Drug Reactions of Imatinib in Patients With Chronic Myeloid Leukemia-A Single-Center Surveillance StudyTazyinul Qoriah AlfauziahNo ratings yet
- Tanya Jawab AqidahDocument44 pagesTanya Jawab AqidahFerdian ZamanNo ratings yet
- Mekanisme Sifat Bakterisidal AminoglikosidaDocument2 pagesMekanisme Sifat Bakterisidal AminoglikosidaTazyinul Qoriah AlfauziahNo ratings yet
- Colour Tests for Drug IdentificationDocument23 pagesColour Tests for Drug IdentificationTazyinul Qoriah AlfauziahNo ratings yet
- Citric Acid Cycle 1: C483 Spring 2013Document18 pagesCitric Acid Cycle 1: C483 Spring 2013Tazyinul Qoriah AlfauziahNo ratings yet
- Mil-Prf-85285e T2 PDFDocument3 pagesMil-Prf-85285e T2 PDFLuis Barrios ArandaNo ratings yet
- Data Daftar Kesetaraan Ijazah Perguruan Tinggi Luar NegeriDocument1,439 pagesData Daftar Kesetaraan Ijazah Perguruan Tinggi Luar NegeriPrabowoNo ratings yet
- ATD ReportDocument17 pagesATD ReportNikhil VajramattiNo ratings yet
- Schedule of Technical Data For Water Tube BoilerDocument5 pagesSchedule of Technical Data For Water Tube BoilerMurugan MuniandyNo ratings yet
- Stereochem Test BankDocument19 pagesStereochem Test BankCarmela Liria100% (15)
- A Textbook of Electrical Technology Vol. 2 - Theraja-P1Document29 pagesA Textbook of Electrical Technology Vol. 2 - Theraja-P1Muhammad TaimoorNo ratings yet
- Epilux 200 Polyamide Cured Coaltar EpoxyDocument3 pagesEpilux 200 Polyamide Cured Coaltar EpoxyIqra AngelsNo ratings yet
- Anti Fouling & Trap Corrosion System (M.G.P.S.) Log SheetDocument2 pagesAnti Fouling & Trap Corrosion System (M.G.P.S.) Log Sheetஎன்றும் அன்புடன் கலைNo ratings yet
- Stoich Paper 2Document56 pagesStoich Paper 2Gangjoon (Ryan) LeeNo ratings yet
- FE00006821 Class VI Injection Permit Salient Features and Regulatory Challenges - FinalDocument65 pagesFE00006821 Class VI Injection Permit Salient Features and Regulatory Challenges - FinalSakshi SinghNo ratings yet
- Aaaa-A (Aaa) - NNNNNN (A) : Piping ClassDocument1 pageAaaa-A (Aaa) - NNNNNN (A) : Piping Classsanjay masoodNo ratings yet
- Heat and Mass Transfer: Fundamentals & ApplicationsDocument59 pagesHeat and Mass Transfer: Fundamentals & ApplicationsabdullahNo ratings yet
- Ch4 PsychrometricsDocument102 pagesCh4 PsychrometricsJayant SisodiaNo ratings yet
- Bonding Types Ionic Covalent MetallicDocument43 pagesBonding Types Ionic Covalent Metallicapi-236069914100% (1)
- Concentration of SolutionsDocument15 pagesConcentration of Solutionsriska raharjoNo ratings yet
- CENTRIFUGATIONDocument21 pagesCENTRIFUGATIONErwin DoloresNo ratings yet
- Denture Base ResinsDocument12 pagesDenture Base ResinsajaytayaNo ratings yet
- SonophoresisDocument32 pagesSonophoresisHiren J PatelNo ratings yet
- Ignition Electrode and Ceramic Igniter Catalog 2020Document26 pagesIgnition Electrode and Ceramic Igniter Catalog 2020FKKNo ratings yet
- 524r - 93 ACI 524R-93 Guide To Portland Cement Plastering PDFDocument28 pages524r - 93 ACI 524R-93 Guide To Portland Cement Plastering PDFSoe Soe100% (1)
- Maxwell 16 Cell LEV DNA Purification Kit ProtocolDocument13 pagesMaxwell 16 Cell LEV DNA Purification Kit ProtocolLinbert Simon CallataNo ratings yet
- ASTM composite standards listDocument6 pagesASTM composite standards listGherlin Kuong67% (3)
- Chapter 21: Electrochemistry: Lesson 21.1: Electrochemical CellsDocument6 pagesChapter 21: Electrochemistry: Lesson 21.1: Electrochemical CellsKM10 khalidNo ratings yet
- Two Film TheoryDocument25 pagesTwo Film TheoryamirulNo ratings yet
- Active Food Packaging. M. L. RooneyDocument293 pagesActive Food Packaging. M. L. RooneyUriel Peña100% (2)
- 1.0tph palm fruite crushing mill-印尼 PDFDocument5 pages1.0tph palm fruite crushing mill-印尼 PDFAgus AriyantoNo ratings yet
- Index TermsDocument90 pagesIndex TermsAlfonso MartínezNo ratings yet
- Barraje Premoldeado Baja TensionDocument2 pagesBarraje Premoldeado Baja TensionPablo SotomayorNo ratings yet
- Delayed Coker Unit PinchDocument28 pagesDelayed Coker Unit PinchPavel MarcinkovicNo ratings yet