Professional Documents
Culture Documents
Homework 11 Solutions
Uploaded by
calebgriffin31Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Homework 11 Solutions
Uploaded by
calebgriffin31Copyright:
Available Formats
Thermodynamics Homework 11 due Friday November 15th
You may complete this assignment individually, or with anyone in your assigned group. Please include both your team number and the ME-IDs of all students who contributed to it. Your team number will only be used to return the assignment to you in your file folder.
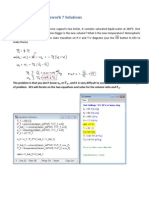
1. An adiabatic piston-cylinder device contains air at 300 K. The air supports 5 large bricks that weigh 40 kg each. The diameter of the piston is 10 cm. Atmospheric pressure is 100 kPa. When all 5 bricks are removed & replaced at once:
The temperature drops from 300 K to 239 K when the bricks are removed and then increases to 409 K when the bricks are returned. The gas did 44 kJ/kg of work when the bricks were removed.
Each brick removed individually
The temperature drops from 300 K to 217 K if all five bricks are removed individually and then increases to 322 K once the bricks are replaced. The gas did a total of 59 kJ/kg of work when the bricks were removed.
Bricks broken up and removed in small pieces (isentropic).
When the bricks are removed in small pieces the temperature dropped from 300 K to 210 K and returns to 300 K when the pieces are returned (the process is reversible). The gas did 65 kJ/kg of work. Discussion The temperature drops increase from removing all 5 bricks at once, removing them one at a time, and removing them in pieces because the process becomes more reversible (more work is done). Similarly, the final temperature is highest when all five bricks are returned at once compared to replacing them one at a time and returning them in pieces. Finally, the most amount of work is done when the bricks are removed in small pieces.
2. An eight-cylinder, four-stroke car engine draws in air at 30 psia and 100F. The cylinders have a bore of 4 in and a stroke of 4.5 in. The enclosed volume in each cylinder is 8 times larger when it is at BDC versus TDC. The flame temperature is 4800 F. Assuming it runs on an ideal Otto cycle, how much power will this engine produce when it is running at 6,000 rpm? Note that if you use the "air" parameter in EES, it is a more accurate calculation because the specific heat varies with temperature, which is more realistic. Either assuming a constant specific heat for air or using EES is fine for these problems.
Compression from 1 to 2 is reversible and adiabatic (isentropic):
(100+459.67)*8^0.4=1,285.784
Expansion from 3 to 4 is isentropic: (4800+459.67)*(1/8)^0.4=2289.4043
0.171*((4800+459.67) - 1286 - 2290 + (100+459.67))=383.6111
(30*8*pi/4*(4/12)^2*(4.5/12)) / (0.3704*(100+459.67)) = 0.0378867083949543
6000/60 * 1/2 * 384 * 0.038 * 1.41 = 1028.736
You might also like
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- Homework 1 SolutionDocument4 pagesHomework 1 Solutioncalebgriffin31No ratings yet
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- Homework 4 SolutionsDocument7 pagesHomework 4 Solutionscalebgriffin31No ratings yet
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Homework 3 SolutionDocument5 pagesHomework 3 Solutioncalebgriffin31No ratings yet
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Homework 2 SolutionDocument4 pagesHomework 2 Solutioncalebgriffin31No ratings yet
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- Homework 5 SolutionsDocument7 pagesHomework 5 Solutionscalebgriffin31No ratings yet
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- Homework 7 SolutionsDocument8 pagesHomework 7 Solutionscalebgriffin31100% (1)
- Homework 8 SolutionsDocument6 pagesHomework 8 Solutionscalebgriffin31No ratings yet
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- Homework 13 SolutionsDocument7 pagesHomework 13 Solutionscalebgriffin31No ratings yet
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Homework 9 SolutionsDocument5 pagesHomework 9 Solutionscalebgriffin31No ratings yet
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- Homework 10 SolutionsDocument8 pagesHomework 10 Solutionscalebgriffin31No ratings yet
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Exam 1 Practice SolutionDocument5 pagesExam 1 Practice Solutioncalebgriffin31100% (1)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- Homework 12 SolutionsDocument6 pagesHomework 12 Solutionscalebgriffin31No ratings yet
- Exam 2 Practice SolutionDocument3 pagesExam 2 Practice Solutioncalebgriffin31100% (1)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- Practice #1-8 SolutionDocument2 pagesPractice #1-8 Solutioncalebgriffin31No ratings yet
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Practice #10 SolutionDocument1 pagePractice #10 Solutioncalebgriffin31No ratings yet
- Final Practice Problems SolutionsDocument9 pagesFinal Practice Problems Solutionscalebgriffin31No ratings yet
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Practice Final Helicopter SolutionDocument1 pagePractice Final Helicopter Solutioncalebgriffin31No ratings yet
- Practice Final Steam Turbine SolutionDocument1 pagePractice Final Steam Turbine Solutioncalebgriffin31No ratings yet
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- Practice Final Conceptual SolutionDocument2 pagesPractice Final Conceptual Solutioncalebgriffin31No ratings yet
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Thermo Final 1Document1 pageThermo Final 1calebgriffin31No ratings yet
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- Thermo Final 2Document1 pageThermo Final 2calebgriffin31No ratings yet
- Gravimetric Methods - 3Document34 pagesGravimetric Methods - 3Anonymous J9kkufNo ratings yet
- HMT Unit 4Document21 pagesHMT Unit 4Muthuvel MNo ratings yet
- Gcse Combined Science - TrilogyDocument20 pagesGcse Combined Science - TrilogyJunaid Asghar0% (1)
- Unit 13 PPT IrDocument21 pagesUnit 13 PPT Irapi-237496924No ratings yet
- University of Santo Tomas CHE 514L: Chemical Engineering Laboratory IIDocument6 pagesUniversity of Santo Tomas CHE 514L: Chemical Engineering Laboratory IIKelly Sheine SisonNo ratings yet
- Chemistry Lab 1Document4 pagesChemistry Lab 1Call Mi BlacksNo ratings yet
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (120)
- Module J: Rubbers As Entropic Springs Example of A Silicone RubberDocument37 pagesModule J: Rubbers As Entropic Springs Example of A Silicone RubberJohn McLovenNo ratings yet
- Title of The Book Thermal TurbomachineryDocument3 pagesTitle of The Book Thermal TurbomachineryPedro LeyvaNo ratings yet
- The Potential and Challenges of Time-Resolved SingDocument33 pagesThe Potential and Challenges of Time-Resolved SingJosh GonzalesNo ratings yet
- Background Information For Ni ComplexDocument3 pagesBackground Information For Ni ComplexKira NguyenNo ratings yet
- Steam Calculation On Basis of Flow-Temperature (1) 2222Document5 pagesSteam Calculation On Basis of Flow-Temperature (1) 2222hmaza shakeelNo ratings yet
- Closed Cell Polyethilene Foam Profile - PRP - Thermoshield-BrochureDocument2 pagesClosed Cell Polyethilene Foam Profile - PRP - Thermoshield-BrochurescubricNo ratings yet
- Diplomarbeit A Versteegh MPI PP Berlin - Kugelblitz PDFDocument123 pagesDiplomarbeit A Versteegh MPI PP Berlin - Kugelblitz PDFtobit3000No ratings yet
- Ce 480 - IoxDocument22 pagesCe 480 - IoxNTEYE CHITONGENo ratings yet
- Humidification DehumidificationDocument31 pagesHumidification DehumidificationyanyanNo ratings yet
- Design of Shell and Tube Heat Exchanger PDFDocument55 pagesDesign of Shell and Tube Heat Exchanger PDFShawez sayyed100% (1)
- DIFFUSION Laboratory ReportDocument2 pagesDIFFUSION Laboratory ReportIsabel Maxine DavidNo ratings yet
- Project Report On Linear Alkyl Benzene Sulfonic AcidDocument79 pagesProject Report On Linear Alkyl Benzene Sulfonic AcidKLNagar seriesNo ratings yet
- BS 5422 2001 Method For Specifying Thermal Insulating Materials For Pipes, Tanks, Vessels, DuctDocument60 pagesBS 5422 2001 Method For Specifying Thermal Insulating Materials For Pipes, Tanks, Vessels, DuctRamiAl-fuqahaNo ratings yet
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- Rheological Behavior of Stabilized Diamond-Graphene Nanoplatelets Hybrid Nanosuspensions in Mineral Oil - ILYAS 2022Document16 pagesRheological Behavior of Stabilized Diamond-Graphene Nanoplatelets Hybrid Nanosuspensions in Mineral Oil - ILYAS 2022gessicapalaoroNo ratings yet
- Statistical Physics by VeselyDocument71 pagesStatistical Physics by VeselycrguntalilibNo ratings yet
- The Three Laws of ThermodynamicsDocument18 pagesThe Three Laws of ThermodynamicsHoney Nhassie Marie GonzagaNo ratings yet
- Chemistry SPM Question Bank Chapter 14Document6 pagesChemistry SPM Question Bank Chapter 14Abdul ManafNo ratings yet
- Rts Using+excelDocument63 pagesRts Using+excelrkibNo ratings yet
- Rtmnu Q Paper Energy Conversion II S 2019Document4 pagesRtmnu Q Paper Energy Conversion II S 2019Sufiyan RehmanNo ratings yet
- Chap. 6Document23 pagesChap. 6Nadeem ShaukatNo ratings yet
- Bimbel 2Document6 pagesBimbel 2Wibowo Sugandi, S.T.No ratings yet
- B.Sc. (Research Notes) ) Self PartII PDFDocument18 pagesB.Sc. (Research Notes) ) Self PartII PDFYash SahajeNo ratings yet
- Characterization of Fabricated MnO2-amberlite Photocatalyst by FTIR, XRD and SEM For Alizarin RemovalDocument6 pagesCharacterization of Fabricated MnO2-amberlite Photocatalyst by FTIR, XRD and SEM For Alizarin RemovalSagir AlvaNo ratings yet
- Identification of Cations Using Ammonium Hydroxide Exp - No.3Document2 pagesIdentification of Cations Using Ammonium Hydroxide Exp - No.3vishal kumarNo ratings yet