Professional Documents
Culture Documents
Thermodynamic
Uploaded by
WanMardziyyahOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Thermodynamic
Uploaded by
WanMardziyyahCopyright:
Available Formats
INTRODUCTION
Do you know that we are surrounded by many kitchen appliances that operate on
the basic principles of physics? The next time you boil water in a whistling
kettle, give a little thought on how the increase in temperature produces steam that
escapes the spout at high pressure.
Inside a pressure cooker, the environment is so tightly sealed that steam is literally
forced through foods. The high pressure inside the cooker also enables cooking
well above 100C. As a result, cooking becomes so much faster and the food is
able to retain most of its nutrients.
Chefs will tell you how meticulous they are about poking holes in potatoes before
baking them. The holes provide an important outlet for the high-pressure steam to
escape; otherwise the potatoes are liable to explode!
T
T
o
o
p
p
i
i
c
c
3
3
TheGasLaws
LEARNING OUTCOMES
By the end of this topic, you should be able to:
1. Understand the concept of the mole;
2. State the relationship between Boltzmanns constant and the gas
constant;
3. State and apply the three gas laws; and
4. State and apply the ideal gas equation.
TOPIC 3 THE GAS LAWS
42
The examples above serve to illustrate the close relationship that exist between
the macroscopic properties of a substance (such as pressure, volume and
temperature) and its mass.
In this topic, we will explore this close relationship with respect to an ideal gas.
THE IDEAL GAS
What is an ideal gas? An ideal gas is any gas that obeys the three ideal gas laws
i.e. Boyles Law, Charles Law and the Pressure Law for all values of pressure P
and temperature T.
3.1.1 The Ideal Gas Law
The ideal gas laws are summarised as follows:
(a) Dependency on the Number of Moles
At constant pressure and temperature, the volume of the gas V is directly
proportional to the number of molecules of the gas N. Since N is large for
gasses, the number of molecules is usually represented by the numbers of
moles n, where
A
N
n
N
= is the ratio for the molecules N with respect to
Avogadros number N
A
. The value of Avogadros number N
A
is 6.02 10
23
molecules/mole. Therefore,
V N n
(3.1)
Also
m
n
M
= is the ratio of the mass of the gas m to the molar mass M.
Molar mass is the mass of a mole of gas and is measured in kg mol
1
.
3.1
TOPIC 3 THE GAS LAWS 43
Example 3.1
Determine the number of moles and the number of molecules of 0.1kg of
nitrogen in a closed container.
Solution
The molar mass of nitrogen is 28 g/mol or 0.028 kg/mol.
23
24
0.1kg
Number of molecules 3.57mole
0.028 kg/mol
Number of molecules (3.57 mole)(6.02 10 molecules/mol)
2.15 10 molecules
A
m
n
M
N nN
= = =
= =
=
(b) Boyles Law
Boyles Law states that the volume V of a gas is inversely proportional to
the pressure P at constant temperature T, i.e.
1
or constant
V
P
PV
=
(3.2)
Thus for a given mass of gas, at constant temperature P
1
V
1
=P
2
V
2
.
The relationship between P and
1
V
is shown in Figure 3.1.
Figure 3.1: Relationship between P and
1
V
How many moles are there in 1 kg of Nitrogen?
(The molar mass of nitrogen is 28 g/mol)
SELF-CHECK 3.1
TOPIC 3 THE GAS LAWS
44
(c) Charles Law
Charles Law states that the volume V of a gas is directly proportional to
the absolute temperature T at constant pressure P.
or constant
V T
V
T
=
(3.3)
Thus for a given gas at constant volume
1 2
1 2
V V
T T
= .
The relationship between V and T is shown in Figure 3.2.
Figure 3.2: Relationship between V and T
(d) The Pressure Law
The Pressure Law states that the pressure P of a gas is directly
proportional to the absolute temperature T at constant volume V, or:
or constant
P T
P
T
=
(3.4)
The relationship between P and T is shown in Figure 3.3.
Figure 3.3: Relationship between P and T
TOPIC 3 THE GAS LAWS 45
3.1.2 The Ideal Gas Equation
From Equations 3.2, 3.3 and 3.4, we can deduce that
constant
PV
T
= (3.5)
Thus, for a certain mass of gas,
1 1 2 2
1 2
PV PV
T T
= .
The ideal gas equation gives the relationship between pressure P, volume V,
temperature T and the number of moles n of a gas in a container, thus
constant =
PV
nT
(3.6)
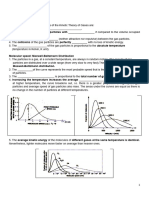
Figure 3.4: The behaviour of gases at high temperature and low pressure
ACTIVITY 3.1
The air pressure in a car tyre increases after driving. Why?
TOPIC 3 THE GAS LAWS
46
This constant, denoted by the letter R, is found to have the same value for all
gases at high T and low P as shown in Figure 3.4. R is called the universal gas
constant with a value equal to 8.315 J mol
1
K
1
.
Thus,
PV
R
nT
=
pV nRT = (3.7)
Equation 3.7 is known as the ideal gas equation.
In practice, there is no such thing as a truly ideal gas! However, it is a very useful
and powerful concept in physics because if the density is low enough all gases
approach the behaviour of an ideal gas. Notice also how we have elegantly
combined the three gas laws into a single equation, known as the ideal gas
equation.
The universal molar ideal gas constant is also given by the product between
Avogadros number N
A
and Boltzmanns constant k, i.e.
R = N
A
k
(3.8)
Therefore the ideal gas equation can also be written as:
PV =nN
A
k T (3.9)
or
PV =Nk T (3.10)
where N = nN
A
is the number of molecules.
Example 3.2
A cylinder of volume 4.0 10
3
m
3
contains gaseous oxygen at a temperature of
27C and pressure of 2 atmospheres. Determine the:
(a) Number of moles of oxygen molecules in the cylinder.
(b) Number of molecules.
TOPIC 3 THE GAS LAWS 47
(c) Mass of the gas.
(d) Density of the gas.
[Given: The molar mass M of oxygen is 0.032 kg mol
1
. 1 atm =1.01 10
5
Pa].
Solution
T =27C =300 K, M =0.032 kg mol
1
=32 g mol
1
(a) Number of moles
5 3
2 1.01 10 4.0 10
0.32moles
8.315 300
PV
n
RT
(b) No. of molecules
23 23
0.32 6.022 10 1.93 10 molecules
A
N nN
(c) Mass 0.32 32 10.24g m nM
(d) Density
3
3
3
10.24 10
2.56kg m
4.0 10
m
V
Example 3.3
What is the volume of a mole of gas at standard temperature and pressure
(S.T.P)?
Solution
The standard pressure is 101.3 kPa and the standard temperature is 273 K.
From pV =nRT
3 3
3
1 8.315 273
22.4 10 m
101.3 10
nRT
V
p
Example 3.4
(a) What volume does 10 g of ammonia gas occupy at S.T.P?
(b) What volume does it occupy at 100C and at pressure 1.5 atm?
Given that the molar mass of ammonia is 17 g/mol.
TOPIC 3 THE GAS LAWS
48
Solution
(a) There are 17 g in one mole of ammonia. So the number of moles in 1 g of
ammonia is therefore
10g
1mole 0.59moles
17g
= .
The volume occupied by 0.058 moles of ammonia at S.T.P can be found by
using the ideal gas equation pV = nRT. At S.T.P, the pressure is 101.3 kPa
and the temperature is 273 K.
So
3 3
3
0.59 8.315 273
13.22 10 m
101.3 10
nRT
V
p
= = =
(b) From
1 1 2 2
1 2
pV p V
T T
=
1 1 2
2
2 1
pVT
V
p T
=
where
3 3
1 1 1
2 2
1atm, 13.22 10 m, 0 C 273K
1.5atm, 100 C 373K
p V T
p T
= = = =
= = =
Substituting the values, we get
2
0.012 V = m.
You should attempt the following questions to test your understanding.
State the differences between macroscopic and microscopic parameters.
In an experiment, it is found that cylinders A and B have equal masses of
gaseous oxygen and nitrogen respectively. Which cylinder has the larger
number of moles?
ACTIVITY 3.2
TOPIC 3 THE GAS LAWS 49
1. Determine the number of moles and number of molecules of 0.1 kg
gaseous oxygen in a container.
2. Determine the volume of 1 mole of an ideal gas at a pressure of
1 atmosphere and temperature of 0C.
3. A cylinder of volume 8.0 10
3
m
3
contains gaseous nitrogen at a
temperature of 27C and pressure of 3 atmospheres. Determine the
(a) Number of moles of nitrogen in the cylinder.
(b) Number of molecules.
(c) Mass of the gas.
(d) Density of the gas if the molar mass of nitrogen is 28 g/mol).
4. A 40 m
3
helium balloon has a temperature of 300 K pressure of
2 atmospheres. The balloon is brought to a height of 6 km and its
pressure and temperature respectively drop to 0.45 atmospheres and
265 K. If the balloon does not burst, what is the volume of the
balloon now?
5. (a) State Boyles law.
(b) An air bubble released at the bottom of a lake expands to
5 times its original volume by the time it reaches the surface. If
the atmospheric pressure is 100 kPa, show that the pressure at
the bottom of the lake is 500 kPa. Assume that the temperature
remains the same.
EXERCISE 3.1
TOPIC 3 THE GAS LAWS
50
XERCISE 3.1
There are three ideal gas laws mentioned in the topic:
- Boyles law;
- Charles law; and
- Pressure law.
The ideal gas equation is pV nRT = .
Boyles law
Charles law
Ideal gas
Macroscopic quantities
Pressure law
The ideal gas equation
The mole
1. State the ideal gas equation.
2. A sealed cylinder with volume 20 litres contains 0.14 kg of gaseous
helium at a temperature of 300 K. Determine the number of moles
and the pressure of the gas. (Molar mass of He =4 g mol
1
)
3. The gas in the cylinder mentioned in (2) is heated at constant
volume until its pressure is doubled. What is the new temperature
of the gas?
EXERCISE 3.2
TOPIC 3 THE GAS LAWS 51
1. According to Boyles Law
A. the pressure of a gas is proportional to the volume at constant
temperature.
B. the pressure of a gas is inversely proportional to the volume at
constant temperature.
C. the pressure of a gas is proportional to the temperature at constant
volume.
D. the volume of an ideal gas is proportional to its temperature at constant
pressure.
2. An ideal gas has a volume of 1 Litre at pressure 1.00 atm and temperature
20C. Find its new pressure when the volume is halved and the
temperature is doubled.
A. 0.81 atm
B. 1.63 atm
C. 2.50 atm
D. 4.00 atm
3. What volume does 20 g of oxygen gas occupy at S.T.P? ( Molar mass of
oxygen is 32 g/mol)
A. 0.009 m
B. 0.014 m
C. 0.018 m
D. 0.022 m
4. Determine the number of molecules in 32 kg of oxygen gas.
A. 6.02 10
23
B. 6.02 10
24
C. 6.02 10
25
D. 6.02 10
26
TOPIC 3 THE GAS LAWS
52
5. A quantity of gas is heated from 300K to 450K at constant pressure. Find the
percentage increase in the volume of the gas
A. 25 %
B. 50 %
C. 75 %
D. 100 %
1. Define the following:
(a) Avogadros number.
(b) The mole.
(c) An ideal gas.
2. Sketch a graph to show the behaviour of gases at high temperatures and at
low pressures.
3. A cylinder of volume 8.0 10
3
m
3
contains an ideal gas at a temperature of
27C and pressure of 4 atmospheres. Determine
(a) The number of moles of oxygen molecules in the cylinder.
(b) The number of gas molecules.
(c) The mass of the gas.
(d) Density of the gas.
[Given: The molar mass of the gas is 40 g mol
1
].
You might also like
- Guide For The Nondestructive Examination of Welds: AWS B1.10M/B1.10:2009 An American National StandardDocument6 pagesGuide For The Nondestructive Examination of Welds: AWS B1.10M/B1.10:2009 An American National StandardRodrigo Paez0% (8)
- UT CH 301 UNIT 1 EXAM-SolutionsDocument7 pagesUT CH 301 UNIT 1 EXAM-SolutionsbrunosipodNo ratings yet
- GLWS9Document6 pagesGLWS9Vince HernándezNo ratings yet
- Ansys ProblemsDocument42 pagesAnsys Problemsmanu198702No ratings yet
- Gas Equations2Document43 pagesGas Equations2api-280572108No ratings yet
- GasesDocument16 pagesGasesAnas MohamedNo ratings yet
- GASESSDocument10 pagesGASESSAndrea Martinez ZepedaNo ratings yet
- Chapter 10 Jan13Document104 pagesChapter 10 Jan13kumuthaNo ratings yet
- Chem 181 Chemistry of GasesDocument15 pagesChem 181 Chemistry of GasesJoey PooleNo ratings yet
- Chemistry-Differeng Laws of GasesDocument7 pagesChemistry-Differeng Laws of GasesMayette TrinidadNo ratings yet
- Gases and Their PropertiesDocument27 pagesGases and Their PropertiesMansonNo ratings yet
- Chemistry Notes Ideal Gas LawsjDocument27 pagesChemistry Notes Ideal Gas LawsjZia RathoreNo ratings yet
- Gaseous StateDocument39 pagesGaseous Statesourabhmaths100% (1)
- 03 Gas ReformatDocument29 pages03 Gas Reformatshanthiny75No ratings yet
- Kinetic Theory of Gas: Concept MapDocument7 pagesKinetic Theory of Gas: Concept MapSoham NagNo ratings yet
- 4.1 GasesDocument23 pages4.1 GasesVasanth Kumar BatumalaiNo ratings yet
- Chapter 5 GasesDocument20 pagesChapter 5 GasesKevin MellizaNo ratings yet
- Behaviour of Perfect Gas Class XIDocument12 pagesBehaviour of Perfect Gas Class XIKAMAL KANT KUSHWAHANo ratings yet
- State of Matter-Gas)Document48 pagesState of Matter-Gas)bigsnailz100% (1)
- Topic 3.2 - Modeling A GasDocument49 pagesTopic 3.2 - Modeling A GasPaul AmezquitaNo ratings yet
- Behavior of Pure Substances: Than One Phase, But Each Phase Must Have The Same Chemical CompositionDocument18 pagesBehavior of Pure Substances: Than One Phase, But Each Phase Must Have The Same Chemical CompositionDharmesh PatelNo ratings yet
- Ch02 (EE)Document135 pagesCh02 (EE)Carl Agape DavisNo ratings yet
- Chapter 4 States of Matter 2021Document24 pagesChapter 4 States of Matter 2021suh mey chongNo ratings yet
- 05 Gases Without AnswersDocument17 pages05 Gases Without Answersapi-287405319No ratings yet
- Gas Laws: Physics IiDocument33 pagesGas Laws: Physics IiEsmeralda Tapiz100% (1)
- LEARNING ACTIVITY SHEET-CHEM 1 q1 Week 4Document8 pagesLEARNING ACTIVITY SHEET-CHEM 1 q1 Week 4Jhude JosephNo ratings yet
- Lesson 4-A2 PhysicsDocument12 pagesLesson 4-A2 PhysicsShiu Ping Wong0% (1)
- SCIENCE WORKSHEET For GRADE 10 Fourth Quarter (WEEK 2)Document3 pagesSCIENCE WORKSHEET For GRADE 10 Fourth Quarter (WEEK 2)Sitti Rohima MarajanNo ratings yet
- KMT and Boyles LawDocument67 pagesKMT and Boyles Lawpandoralistik1No ratings yet
- 01 The Gas Laws-Complete STDocument55 pages01 The Gas Laws-Complete STRyan RamlawiNo ratings yet
- Avogadro's LawDocument5 pagesAvogadro's LawHeng Pris100% (1)
- Gases (Chapter 5) : (1.00 Mol) (0.08206 Mol K L Atm) (273 K) 22.414 LDocument11 pagesGases (Chapter 5) : (1.00 Mol) (0.08206 Mol K L Atm) (273 K) 22.414 LHemant KumarNo ratings yet
- Phys2 Ch4 Kineticsgas NewDocument76 pagesPhys2 Ch4 Kineticsgas NewQuỳnh NguyễnNo ratings yet
- Gas Laws LecDocument43 pagesGas Laws LecJune Francis AngNo ratings yet
- Topic 1 - Stoichiometric Relationships - Part 2Document15 pagesTopic 1 - Stoichiometric Relationships - Part 2burcak gecNo ratings yet
- Physical Chemistry Author DR Hasan MaridiDocument78 pagesPhysical Chemistry Author DR Hasan MaridiAbinow SNo ratings yet
- 5 Temp Ideal Gas-Fall 2022Document22 pages5 Temp Ideal Gas-Fall 2022asakr8481No ratings yet
- Engineering Chemistry 1Document49 pagesEngineering Chemistry 1Rasha HajaratNo ratings yet
- ChE 323 Prob Set 1 11 - 30-13Document2 pagesChE 323 Prob Set 1 11 - 30-13Stephanie JainarNo ratings yet
- IAS Calcuating Molar Volumes and Ideal Gas BehaviourDocument18 pagesIAS Calcuating Molar Volumes and Ideal Gas BehaviourNico Van De CasteeleNo ratings yet
- The Kinetic Molecular Theory: General Chemistry 1 Reviewer: 2nd QuarterDocument15 pagesThe Kinetic Molecular Theory: General Chemistry 1 Reviewer: 2nd QuarterJerome jeromeNo ratings yet
- Measurement 16Document16 pagesMeasurement 16Gaurav ShekharNo ratings yet
- Unit 3 Assignment AnswersDocument0 pagesUnit 3 Assignment AnswersRosanna LombresNo ratings yet
- AvocadoDocument8 pagesAvocadoKaye GelieNo ratings yet
- State of MatterDocument52 pagesState of MatterAditi MahajanNo ratings yet
- 1.23 Gas Calculations: Molar Gas Volume (OCR +EDEXCEL)Document7 pages1.23 Gas Calculations: Molar Gas Volume (OCR +EDEXCEL)SunnyNo ratings yet
- Program of "Physics": Lecturer: Dr. DO Xuan Hoi Room 413 E-MailDocument78 pagesProgram of "Physics": Lecturer: Dr. DO Xuan Hoi Room 413 E-MailJamir EscalanteNo ratings yet
- Chemistry Form 6 Sem 1 04Document64 pagesChemistry Form 6 Sem 1 04Ng Swee Loong Steven100% (6)
- Kinetic Theory of Gases PresentationDocument13 pagesKinetic Theory of Gases PresentationF F ID KingNo ratings yet
- Gas Laws:: P V K VDocument18 pagesGas Laws:: P V K VFarah Zu'biNo ratings yet
- Gas LawDocument16 pagesGas LawmillergraNo ratings yet
- Ideal Gas: General Chemistry 1Document9 pagesIdeal Gas: General Chemistry 1Daniel Corcino100% (1)
- AIR QUALITY AND POLLUTION (TKA 3301) LECTURE NOTES 4-Chemistry of Air Pollution N Ideal Gas LawDocument66 pagesAIR QUALITY AND POLLUTION (TKA 3301) LECTURE NOTES 4-Chemistry of Air Pollution N Ideal Gas Lawmamat88No ratings yet
- NCERT Solutions For Class 11 Physics 15may Chapter 13 Kinetic TheoryDocument15 pagesNCERT Solutions For Class 11 Physics 15may Chapter 13 Kinetic TheoryGSN KISHORENo ratings yet
- GasDocument26 pagesGasJoshua PhillippsNo ratings yet
- WS Practice W GraphsDocument4 pagesWS Practice W GraphsgiyagirlsNo ratings yet
- Chapter 5 - The Gaseous StateDocument33 pagesChapter 5 - The Gaseous StateRashid EmoroniNo ratings yet
- The Gaseous State: The Commonwealth and International Library: Chemistry DivisionFrom EverandThe Gaseous State: The Commonwealth and International Library: Chemistry DivisionNo ratings yet
- Heat and TemperatureDocument26 pagesHeat and TemperatureWanMardziyyahNo ratings yet
- EXPERIMENT Density of SolidDocument4 pagesEXPERIMENT Density of SolidWanMardziyyahNo ratings yet
- Lesson Plan Worksite Hazard Analysis 1-Hour ModuleDocument2 pagesLesson Plan Worksite Hazard Analysis 1-Hour ModuleWanMardziyyahNo ratings yet
- September Semester 2010 Ebmp3103/Edmp3103 - Production Technology Assignment (30%)Document5 pagesSeptember Semester 2010 Ebmp3103/Edmp3103 - Production Technology Assignment (30%)WanMardziyyahNo ratings yet
- Optical Communications4Document19 pagesOptical Communications4keane1No ratings yet
- W3SZ PackRatsConference2014Document16 pagesW3SZ PackRatsConference2014Dominic Mendoza100% (1)
- 316 316L Technical Information SheetDocument5 pages316 316L Technical Information SheetfejlongNo ratings yet
- What Is The Ozone Layer and Where Is It Located?Document6 pagesWhat Is The Ozone Layer and Where Is It Located?JoseNo ratings yet
- Response Spectrum Calculations Using Ubc 97: Ifzoneis4Document3 pagesResponse Spectrum Calculations Using Ubc 97: Ifzoneis4maheshNo ratings yet
- Lecture 3 - Curves in Space and Their TangentDocument38 pagesLecture 3 - Curves in Space and Their TangentNURUL YAHSIFAH SYQELLA BINTI YAHYA BK21110100No ratings yet
- E201: Work, Energy and Power POLICIOUS, Mark Angelo FDocument5 pagesE201: Work, Energy and Power POLICIOUS, Mark Angelo FMark Angelo PoliciosNo ratings yet
- EEE-ETI 3208 ELECTROMAGNETICS III ExamDocument3 pagesEEE-ETI 3208 ELECTROMAGNETICS III ExamHenry Kabasa100% (1)
- Dew Point Calculation Chart: For Adhesive and Coating Applications Ambient Air Temperature in Degrees FahrenheitDocument1 pageDew Point Calculation Chart: For Adhesive and Coating Applications Ambient Air Temperature in Degrees FahrenheitBernathTurnipNo ratings yet
- Beniston (2006) Mountain Weather and Climate A General Overview and A Focus On Climatic Change in The AlpsDocument14 pagesBeniston (2006) Mountain Weather and Climate A General Overview and A Focus On Climatic Change in The AlpspierinaNo ratings yet
- Thermal Stability and Performance Data For Sm-Co 2 17 High-TemperatureDocument4 pagesThermal Stability and Performance Data For Sm-Co 2 17 High-TemperatureelectronenergyNo ratings yet
- The HALO Range: Uv-Visible and Visible SpectrophotometersDocument9 pagesThe HALO Range: Uv-Visible and Visible Spectrophotometersrohmen042No ratings yet
- Pump Jack, PumpsDocument8 pagesPump Jack, PumpsТатьяна НестеренкоNo ratings yet
- LYX100 ManualDocument12 pagesLYX100 ManualEMMANo ratings yet
- Ball Joint Failure DocumentDocument2 pagesBall Joint Failure DocumentAbeer Darhous100% (1)
- RV College of EngineeringDocument12 pagesRV College of EngineeringRahul ChandraNo ratings yet
- Application of Sound Power LevelDocument8 pagesApplication of Sound Power LevelmanchuricoNo ratings yet
- B146.01 1-EnDocument67 pagesB146.01 1-EnLoed DeolNo ratings yet
- ch14 1Document7 pagesch14 1jiholee1117No ratings yet
- Solidification of Iron Castings - 314983Document1 pageSolidification of Iron Castings - 314983Ankur PatelNo ratings yet
- Achieve™ Advanced PP8285E1: Polypropylene Impact CopolymerDocument2 pagesAchieve™ Advanced PP8285E1: Polypropylene Impact CopolymermosesNo ratings yet
- List of NDT EquipmentsDocument1 pageList of NDT EquipmentsAnandNo ratings yet
- Manual de Operación Mini Sapray Dryer b290Document57 pagesManual de Operación Mini Sapray Dryer b290Gustavo MoralesNo ratings yet
- Charny - Mathematical Models of Bioheat TransferDocument137 pagesCharny - Mathematical Models of Bioheat TransferMadalena PanNo ratings yet
- Pages From 0625 - s16 - QP - 42 - 01Document2 pagesPages From 0625 - s16 - QP - 42 - 01lelon ongNo ratings yet
- Tugas Strategi Rencana ProsesDocument3 pagesTugas Strategi Rencana ProsesgeafitriaNo ratings yet
- Desmi Pumps PDFDocument30 pagesDesmi Pumps PDFlunatic20000% (1)
- M.S. Abd-ElhadyDocument8 pagesM.S. Abd-ElhadyNasser93No ratings yet