0% found this document useful (1 vote)
1K views3 pagesStereochemistry Worksheet Lab
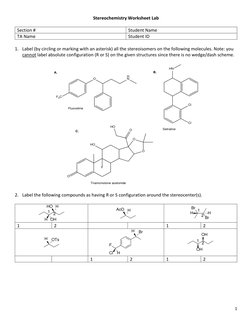
This document is a stereochemistry worksheet that contains questions about:
1) Labeling stereoisomers in molecules
2) Assigning R/S configuration to stereocenters
3) Providing examples of stereoisomer types
4) Drawing chiral molecules with multiple stereocenters
5) Completing stereochemical reactions and determining product relationships
6) Drawing mechanisms for stereochemical reactions involving substitution, elimination, and addition.
Uploaded by
Daniel McDermottCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as PDF, TXT or read online on Scribd
0% found this document useful (1 vote)
1K views3 pagesStereochemistry Worksheet Lab
This document is a stereochemistry worksheet that contains questions about:
1) Labeling stereoisomers in molecules
2) Assigning R/S configuration to stereocenters
3) Providing examples of stereoisomer types
4) Drawing chiral molecules with multiple stereocenters
5) Completing stereochemical reactions and determining product relationships
6) Drawing mechanisms for stereochemical reactions involving substitution, elimination, and addition.
Uploaded by
Daniel McDermottCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as PDF, TXT or read online on Scribd
- Stereochemistry Worksheet Lab: Worksheet exercises focused on labeling stereoisomers and identifying stereochemical configurations in molecules.