Professional Documents
Culture Documents
Max Max Max Max Max Max
Uploaded by
mkmr484Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Max Max Max Max Max Max
Uploaded by
mkmr484Copyright:
Available Formats
Procedure 1.
1.) Using a glass graduated cylinder, measure 9 mL of deionized water and pour it
into the vial.
2.) Using a plastic syringe, add 1.0 mL of 1.0 mM AgNO3 to the glass vial.
3.) Add 0.6 mL of 0.7 mM PVP using a plastic syringe into the vial.
4) Add 0.6 mL of 3 mM sodium citrate using a plastic syringe into the vial.
4.) Add 0.25 mL of 3% H2O2 using a plastic syringe in the same vial.
5.) Put the cap on the vial and invert several times in order to mix the reagents
completely.
6.) Check with your teacher to determine how much of the 10 mM NaBH4 to add.
Quickly cap the vial and begin to shake vigorously. (Record the amount of NaBH4
added to the vial.)
7.) The solution should turn a very pale yellow color at first (A good indicator that
the synthesis is proceeding well). After shaking the vial for additional 5-10
minute, something very interesting should occur. Continue to shake for a full two
minutes after the change occurs. Make sure to record your observations
thoroughly.
Amount of Sodium Borohydride Added (mL) Final Color Attained
0.3 Yellow (max ~ 465 nm)
0.32 Light pink (max ~ 505 nm)
0.35 Darker Pink (max ~ 510 nm)
0.4 Violet (max ~ 545)
0.45 Blue/Purple (max ~ 615)
0.5 Blue (max ~ 650)
*Blue and yellow solutions could be combined to obtain a green solution.
You might also like
- Science IV 2011-2012Document9 pagesScience IV 2011-2012Marc SealzaNo ratings yet
- Animal Protein Identification Test Method-3Document1 pageAnimal Protein Identification Test Method-3mangala jesudossNo ratings yet
- Krishnapriya ReportDocument9 pagesKrishnapriya ReportKrishnapriya ThakurNo ratings yet
- Effect of pH and Temperature on Salivary Digestion of StarchDocument9 pagesEffect of pH and Temperature on Salivary Digestion of StarchAnkush Dhingra100% (1)
- ChemistryDocument11 pagesChemistryapi-327825016No ratings yet
- The Blue Bottle' Experiment: Topic Timing Level DescriptionDocument2 pagesThe Blue Bottle' Experiment: Topic Timing Level DescriptionRodrigo Souza BanegasNo ratings yet
- DNA ExtractionDocument2 pagesDNA ExtractionRouse Leanne NicolasNo ratings yet
- Project Digestion of Starch Class 12Document15 pagesProject Digestion of Starch Class 12Shreyash PolNo ratings yet
- Photosynthesis LabDocument3 pagesPhotosynthesis LabChloe Gloria0% (1)
- Magic of ChemistryDocument8 pagesMagic of ChemistryFadya Syahnariza Nan BarenoNo ratings yet
- Winkler Method Lab ReportDocument6 pagesWinkler Method Lab ReportYoonseo (Elin) ChaNo ratings yet
- ALENGOSVIG's HowTo Upped by Met A FractalDocument15 pagesALENGOSVIG's HowTo Upped by Met A FractalPhileasNo ratings yet
- Cfns Experiment 48 - The Blue Bottle ExperimentDocument2 pagesCfns Experiment 48 - The Blue Bottle ExperimentJPNo ratings yet
- Confounding Color: What You NeedDocument9 pagesConfounding Color: What You NeedAnonymous hb3Dvgc7No ratings yet
- BiolabreportDocument9 pagesBiolabreportapi-276911762No ratings yet
- Presentation ChemistryDocument14 pagesPresentation ChemistryDIWAKER ojha X 'A'No ratings yet
- CombineDocument2 pagesCombineapi-254428474No ratings yet
- EXercise 2 (Recrystallization and Melting Point Determination)Document3 pagesEXercise 2 (Recrystallization and Melting Point Determination)fangirltonNo ratings yet
- GCSE Combined RP Methodology + Video LinksDocument6 pagesGCSE Combined RP Methodology + Video Linkszhinia HossainNo ratings yet
- Determination of Moisture Content (AOAC 1970) : Flowchart Attachment 1Document8 pagesDetermination of Moisture Content (AOAC 1970) : Flowchart Attachment 1Zulvava AbidahNo ratings yet
- Aswika Project ContentDocument7 pagesAswika Project ContentRoshini SNo ratings yet
- Enzyme Lab Report - Leonarda MaticDocument12 pagesEnzyme Lab Report - Leonarda MaticlmaticNo ratings yet
- Biology lab report on organic cell compositionsDocument10 pagesBiology lab report on organic cell compositionsNgọc Phương Anh NguyễnNo ratings yet
- Lab 03 Crystallization of An UnknownDocument7 pagesLab 03 Crystallization of An UnknownReyNo ratings yet
- Lab Report CrystallizationDocument5 pagesLab Report Crystallizationapi-334673900No ratings yet
- TRO MFGDocument2 pagesTRO MFGJalindar BansodeNo ratings yet
- Bio Lab 2Document7 pagesBio Lab 2CassyNo ratings yet
- UV-Vis SOP for Sugar AnalysisDocument2 pagesUV-Vis SOP for Sugar AnalysisZaid YahyaNo ratings yet
- DNA Extraction SOPDocument15 pagesDNA Extraction SOPattiyaNo ratings yet
- To Study The Digestion of Starch by SaliDocument13 pagesTo Study The Digestion of Starch by SaliMihira BhaleraoNo ratings yet
- Chemistry PRJCTDocument17 pagesChemistry PRJCTVansh BansalNo ratings yet
- Organic Chemistry Exp.3Document3 pagesOrganic Chemistry Exp.3Aws SarajNo ratings yet
- Lab ReportDocument19 pagesLab Reportapi-394241963100% (1)
- Experiment #1:: Purification of Benzoic Acid by RecrystallizationDocument51 pagesExperiment #1:: Purification of Benzoic Acid by RecrystallizationStephanie Ann Marie DueñasNo ratings yet
- Common Laboratory TechniquesDocument3 pagesCommon Laboratory TechniquesYannie GomezNo ratings yet
- MMMJ PDFDocument16 pagesMMMJ PDFmansurimuaaj099No ratings yet
- Experiment-No.-6_Saponification-1Document2 pagesExperiment-No.-6_Saponification-1maicadicionNo ratings yet
- Isolating Pseudo EphedrineDocument4 pagesIsolating Pseudo EphedrineJosh Roesler80% (5)
- Lab Act - 2 ENZYMESDocument3 pagesLab Act - 2 ENZYMESJhia TorreonNo ratings yet
- List of ExperimentsDocument15 pagesList of ExperimentsSeetharam RaoNo ratings yet
- Chemistry ProjectDocument9 pagesChemistry ProjectbanojitmallickNo ratings yet
- Experiment 4Document20 pagesExperiment 4William Allan Arcilla100% (3)
- Effect of temperature and pH on salivary digestion of starchDocument12 pagesEffect of temperature and pH on salivary digestion of starchsaurabh rockNo ratings yet
- Identify Macromolecules Using Qualitative TestsDocument4 pagesIdentify Macromolecules Using Qualitative TestsPam SNo ratings yet
- Activity 3 4 Chem Lab PilareDocument8 pagesActivity 3 4 Chem Lab PilareJushelle Anne Tigoy PilareNo ratings yet
- LB Agar RecipeDocument1 pageLB Agar RecipeStrahinja SkoboNo ratings yet
- Lap Report Cu Oh 2Document14 pagesLap Report Cu Oh 2api-389948390No ratings yet
- Re CrystallizationDocument7 pagesRe CrystallizationAli NasrallahNo ratings yet
- Gravimetric Analysis of SULFATEDocument2 pagesGravimetric Analysis of SULFATEIsrael Lopez Kahlo100% (2)
- Recrystallization NotesDocument2 pagesRecrystallization NotesSonya SandhuNo ratings yet
- EXP2 BIOCHEM Analyzing and Determine Sugars and Starch in Plant Tissues.Document10 pagesEXP2 BIOCHEM Analyzing and Determine Sugars and Starch in Plant Tissues.NUR AMALIA BINTI MAZLEE STUDENTNo ratings yet
- LugolDocument6 pagesLugolJhon carlo CastroNo ratings yet
- BIO303 Biochemistry IIDocument38 pagesBIO303 Biochemistry IIHanifullah JanNo ratings yet
- Lab Report 2Document8 pagesLab Report 2Bùi Nhật MaiNo ratings yet
- Chemistry Class 12 ProjectDocument10 pagesChemistry Class 12 ProjectsaransasthiNo ratings yet
- Biology Lab Report Organic MoleculesDocument5 pagesBiology Lab Report Organic Moleculesapi-257306447No ratings yet
- Home Comforts: The Art of Transforming Your Home Into Your Own Personal ParadiseFrom EverandHome Comforts: The Art of Transforming Your Home Into Your Own Personal ParadiseNo ratings yet
- Institute of Nano Science and Technology: Form For Purchase of Goods Under GFR 145Document1 pageInstitute of Nano Science and Technology: Form For Purchase of Goods Under GFR 145mkmr484No ratings yet
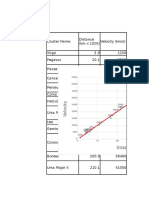
- Velocity Vs DistanceDocument2 pagesVelocity Vs Distancemkmr484No ratings yet
- Registration Form CRIKC NanoDocument1 pageRegistration Form CRIKC Nanomkmr484No ratings yet
- Lab Beers LawDocument5 pagesLab Beers LawNurul AzizahNo ratings yet
- Angular MomentumDocument3 pagesAngular Momentummkmr484No ratings yet
- IISER Mohali PhD student course registration formDocument1 pageIISER Mohali PhD student course registration formmkmr484No ratings yet
- Product Information: LA653 Dialysis Tubing Size 1 Dia 8/32, 6.3 MMDocument1 pageProduct Information: LA653 Dialysis Tubing Size 1 Dia 8/32, 6.3 MMmkmr484No ratings yet
- Himedia Products Catalogue 2012-13: Rapid Rapid Rapid Rapid Rapid Rapid RapidDocument1 pageHimedia Products Catalogue 2012-13: Rapid Rapid Rapid Rapid Rapid Rapid Rapidmkmr484No ratings yet
- Fee AjitDocument1 pageFee Ajitmkmr484No ratings yet
- AjitDocument3 pagesAjitmkmr484No ratings yet
- DBTIndi May2017 2Document1 pageDBTIndi May2017 2mkmr484No ratings yet
- Full TextDocument28 pagesFull Textmkmr484No ratings yet
- PH 2Document2 pagesPH 2mkmr484No ratings yet
- Derivation of Physical Parameters From Raman Spectra of Hard Carbon FilmsDocument4 pagesDerivation of Physical Parameters From Raman Spectra of Hard Carbon Filmsmkmr484No ratings yet
- TyndalDocument1 pageTyndalmkmr484No ratings yet
- DBTProformaDocument3 pagesDBTProformamkmr484No ratings yet
- Derivation of Physical Parameters From Raman Spectra of Hard Carbon FilmsDocument4 pagesDerivation of Physical Parameters From Raman Spectra of Hard Carbon Filmsmkmr484No ratings yet
- DBTProformaDocument3 pagesDBTProformamkmr484No ratings yet
- DBTProformaDocument3 pagesDBTProformamkmr484No ratings yet
- DBTProformaDocument3 pagesDBTProformamkmr484No ratings yet
- DBTProformaDocument3 pagesDBTProformamkmr484No ratings yet
- Time Table For Even-Sem INST Courses Jan-Apr 2017Document2 pagesTime Table For Even-Sem INST Courses Jan-Apr 2017mkmr484No ratings yet
- Time Table For Even-Sem INST Courses Jan-Apr 2017Document2 pagesTime Table For Even-Sem INST Courses Jan-Apr 2017mkmr484No ratings yet