Professional Documents
Culture Documents
Pka Chart: Conjugate Acid Conjugate Acid
Pka Chart: Conjugate Acid Conjugate Acid
Uploaded by
_MPDOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Pka Chart: Conjugate Acid Conjugate Acid
Pka Chart: Conjugate Acid Conjugate Acid
Uploaded by
_MPDCopyright:
Available Formats
pKa Chart 2
1
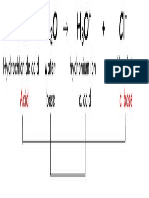
conjugate acid conjugate base conjugate acid conjugate base
strongest acids
weakest bases
sulfuric acid H2SO4 HSO4 -10 nitric acid HNO3 -1.3
NO3
hydroiodic acid HI -9
I tosic acid TsOH TsO- -0.6
hydrobromic acid HBr Br -8 hydrofluoric acid 3.2
HF F
H
O O
protonated
-7.3
ketone hydrogen nitride HN3 N3 4.7
hydrochloric acid HCl Cl -7
O O
carboxylic acids H 4.8
O O
protonated O O -3.6
ether
H
H
protonated
pyridine 5.2
carbocations -3 NH N
carbonic acid H2CO3 HCO3 6.37
protonated H H
alcohol O O
-2.4 (bicarbonate)
H
hydrogen sulfide H2S SH 7
O
hydronium ion H H O -1.7
H H H
3 4
conjugate acid conjugate base conjugate acid conjugate base
O O O O water O
O H 15.7
H H
1,3-diketones 9 (hydroxide)
primary alcohols H 16
hydrogen cyanide H C N C N 9.1 O O
(cyanide) (alkoxides)
H
ammonium ions N 9.4 tertiary alcohols
H N H H H O O 18
H
H H (tert-butoxide)
O O
OH O
phenols 10 ketones 18-21
(enolates)
O O
bicarbonate HCO3 - -2
CO3 10.25 esters 25
O O
(ester enolates)
H 10.5 26
thiols S S alkynes C C H C C
(acetylide anions)
H H
protonated H
amines N H3C N 10.6 hydrogen H H H (hydride) 35
H3C H H one way only
H
H N
cyclopentadiene 15 ammonia/amines N 36
weakest acids
H R H R H
strongest bases
H
(aromatic) (amide bases)
O O
amides 15 alkanes ~60
NH2 NH H one way only MgBr
(Grignards/ organolithium reagents)
You might also like
- Common Chemical Formula ListDocument3 pagesCommon Chemical Formula Listaran9283% (6)
- Ch18Acid Base (A)Document45 pagesCh18Acid Base (A)Sam H. SalehNo ratings yet
- Acid and Base Strengths: A B B CDocument1 pageAcid and Base Strengths: A B B CVIKAS GARGNo ratings yet
- Acizi Si BazeDocument4 pagesAcizi Si BazeKristanna123No ratings yet
- Weak Acids, Weak Bases and BuffersDocument23 pagesWeak Acids, Weak Bases and BuffersCansın UğurNo ratings yet
- Pka TableDocument1 pagePka TableEman BadryNo ratings yet
- Base Ionization Constants Substance Formula KDocument5 pagesBase Ionization Constants Substance Formula KKhelly Shan C. Sta. RitaNo ratings yet
- Acids&BasesDocument3 pagesAcids&BasesJosh CatolinNo ratings yet
- Acidity Constants Data Part 1Document2 pagesAcidity Constants Data Part 1AceNo ratings yet
- CHPT 16Document12 pagesCHPT 16Duaa RajaNo ratings yet
- Table of Pka ValuesDocument3 pagesTable of Pka ValuesjVNo ratings yet
- The Table of Acid and BaseDocument3 pagesThe Table of Acid and BaseDimas MuhamadNo ratings yet
- Notes Solutions Chapter 07Document15 pagesNotes Solutions Chapter 07Syllvia SunnivaNo ratings yet
- Constantes de Equilibrioacido-Base (25 C) : Acid Formula N Pk1 Pk2 Pk3 Pk4Document2 pagesConstantes de Equilibrioacido-Base (25 C) : Acid Formula N Pk1 Pk2 Pk3 Pk4Naty AvalosNo ratings yet
- 51 HMW Solutions Ch14Document43 pages51 HMW Solutions Ch14Cbn NoonNo ratings yet
- Acids and Bases NotesDocument17 pagesAcids and Bases NotesNap DoNo ratings yet
- Video NotesDocument45 pagesVideo Notesjim tannerNo ratings yet
- Acid BasesDocument2 pagesAcid BasesseruNo ratings yet
- An Incomplete Polyatomic Ion ChartDocument1 pageAn Incomplete Polyatomic Ion ChartGAT TutoringNo ratings yet
- Acid-Base Equilibrium: See Aqueousions in Chemistry 1110 Online Notes For Review of Acid-Base Fundamentals!Document31 pagesAcid-Base Equilibrium: See Aqueousions in Chemistry 1110 Online Notes For Review of Acid-Base Fundamentals!Janna EchavezNo ratings yet
- Lecture 5 Acids and BasesDocument39 pagesLecture 5 Acids and BasesAllen SiaNo ratings yet
- 3.ii. BASES AND ACIDS OF CHOICEDocument110 pages3.ii. BASES AND ACIDS OF CHOICEKeith OmwoyoNo ratings yet
- Conjugate Acid and Base PairsDocument1 pageConjugate Acid and Base Pairsfelisita wisangNo ratings yet
- HSC Chemistry Lesson Plan 16Document8 pagesHSC Chemistry Lesson Plan 16Ali HaidarNo ratings yet
- Chapter 3 Acid - BaseDocument98 pagesChapter 3 Acid - BaseKhoa Nguyen Viet DangNo ratings yet
- Phenol SDocument32 pagesPhenol SBangaloredNo ratings yet
- Acid and BaseDocument2 pagesAcid and BaseMaraNo ratings yet
- Ch14 Study QuestionsDocument3 pagesCh14 Study QuestionsКанат ТютеновNo ratings yet
- PPP1A 8.1 Arrhenius and NamingDocument13 pagesPPP1A 8.1 Arrhenius and NamingRichard LindemannNo ratings yet
- Chapter 3 Acid - BaseDocument98 pagesChapter 3 Acid - BasePHƯƠNG ĐẶNG YẾNNo ratings yet
- Chapter 3 Acid - BaseDocument98 pagesChapter 3 Acid - BaseNhan PhướcNo ratings yet
- Asam LemahDocument2 pagesAsam LemahAddeSRNo ratings yet
- Solutions 222Document8 pagesSolutions 222estellasr00No ratings yet
- Acid Base Notes (Use For Chem 123)Document16 pagesAcid Base Notes (Use For Chem 123)Nate JamesNo ratings yet
- Acid Equilibrium and PH: Søren SørensenDocument16 pagesAcid Equilibrium and PH: Søren Sørensenmonster40lbsNo ratings yet
- New 104 Nucleophilic SubstDocument11 pagesNew 104 Nucleophilic SubstEva PannemanNo ratings yet
- Table of Ka and KBDocument1 pageTable of Ka and KBkarimhandoyoNo ratings yet
- Chapter 3Document124 pagesChapter 3Fariz SharudinNo ratings yet
- Lecture# PhenolDocument16 pagesLecture# PhenolRao Wazim AkramNo ratings yet
- PhenolsDocument34 pagesPhenolsLoran Prelya TengayNo ratings yet
- Natural Produc Chemistry - Alkaloid Biosynthesis - Nila HudaDocument23 pagesNatural Produc Chemistry - Alkaloid Biosynthesis - Nila HudaNilaHudaBaqirNo ratings yet
- Asam BasaDocument13 pagesAsam Basaaliefyan4769No ratings yet
- Name: KEY Nomenclature - Covalent (Molecular) Compounds Part A: Name The Following Covalent Compounds. Part B: Write The Chemical Formula For Each of The Following CompoundsDocument2 pagesName: KEY Nomenclature - Covalent (Molecular) Compounds Part A: Name The Following Covalent Compounds. Part B: Write The Chemical Formula For Each of The Following CompoundsTrung LuongNo ratings yet
- 1288 PhenolsDocument29 pages1288 PhenolsX ThrxNo ratings yet
- Activity 2.1 - Acids and Bases NMNMDocument3 pagesActivity 2.1 - Acids and Bases NMNMClarise CanANo ratings yet
- Chapter 6-Acid and Base PDFDocument47 pagesChapter 6-Acid and Base PDFWhafimsNo ratings yet
- Chapter 3 Acid - BaseDocument96 pagesChapter 3 Acid - BaseAnh NhamNo ratings yet
- Chapter 18 Silberberg AnswersDocument51 pagesChapter 18 Silberberg AnswersKevin DashNo ratings yet
- EMAN YASSIN - Chemistry 12 Unit 4 PacketDocument11 pagesEMAN YASSIN - Chemistry 12 Unit 4 PacketSherine GamalNo ratings yet
- Physical Chemistry QuestionsDocument2 pagesPhysical Chemistry QuestionsClinton Softleigh JrNo ratings yet
- 7.0 Ionic Equilibria (Students)Document187 pages7.0 Ionic Equilibria (Students)Supia Nazma100% (1)
- Acids and Bases Note SapDocument30 pagesAcids and Bases Note SapNabilah MustafaNo ratings yet
- Acids Bases SaltsDocument11 pagesAcids Bases Saltsabiodun olaokeNo ratings yet
- Acids BasesDocument30 pagesAcids BasesHaniel GalzoteNo ratings yet
- Recent Developments in the Chemistry of Natural Phenolic Compounds: Proceedings of the Plant Phenolics Group SymposiumFrom EverandRecent Developments in the Chemistry of Natural Phenolic Compounds: Proceedings of the Plant Phenolics Group SymposiumW. D. OllisNo ratings yet
- Monohydric Alcohols Their Ethers and Esters Sulphur Analogues Nitrogen Derivatives Organometallic Compounds: A Modern Comprehensive TreatiseFrom EverandMonohydric Alcohols Their Ethers and Esters Sulphur Analogues Nitrogen Derivatives Organometallic Compounds: A Modern Comprehensive TreatiseNo ratings yet
- Aliphatic Compounds: A Modern Comprehensive TreatiseFrom EverandAliphatic Compounds: A Modern Comprehensive TreatiseNo ratings yet
- "And, When You Want Something, ALL The: UniverseDocument1 page"And, When You Want Something, ALL The: UniverseSayNo ratings yet
- Gases AnswersDocument8 pagesGases AnswersSayNo ratings yet
- Key 3Document7 pagesKey 3SayNo ratings yet
- Social Science Nmat ReviewerDocument13 pagesSocial Science Nmat ReviewerSay77% (13)