Professional Documents
Culture Documents
Barrangou Et Al. - 2007 - CRISPR Provides Acquired Resistance Against Viruses in Prokaryotes PDF
Uploaded by
flashjetOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Barrangou Et Al. - 2007 - CRISPR Provides Acquired Resistance Against Viruses in Prokaryotes PDF
Uploaded by
flashjetCopyright:
Available Formats
REPORTS
Fig. 3. Drag coefficient as x 10
−3 4. M. D. Powell, P. J. Vickery, T. A. Reinhold, Nature 422,
4 279 (2003).
a function of wind speed. CD
5. E. D. Fernandez et al., J. Geophys. Res. 111, C08013
is shown for an observation- 10.1029/2005JC003048 (2006).
based resistance coefficient, 6. I. J. Moon, I. Ginis, T. Hara, J. Atmos. Sci. 61, 2334 (2004).
r = 0.02 cm s−1. The red 7. J. A. T. Bye, A. D. Jenkins, J. Geophys. Res. 111, C03024
open circles are the eval- 3 10.1029/2005JC003114 (2006).
uated CD from the current 8. K. Emanuel, J. Atmos. Sci. 60, 1420 (2003).
and wind observations, the 9. D. A. Mitchell, W. J. Teague, E. Jarosz, D. W. Wang,
Drag Coefficient (C )
D
Geophys. Res. Lett. 32, L11610 10.1029/2005GL023014
solid red line is a fitted (2005).
quadratic curve to the CD 10. D. W. Wang, D. A. Mitchell, W. J. Teague, E. Jarosz,
2
estimates, and the red M. S. Hulbert, Science 309, 896 (2005).
dashed lines are the 95% 11. W. J. Teague, E. Jarosz, D. W. Wang, D. A. Mitchell,
confidence limits for this J. Phys. Oceanogr., in press.
12. W. J. Teague, E. Jarosz, M. R. Carnes, D. A. Mitchell,
quadratic curve. The black
P. J. Hogan, Cont. Shelf Res. 26, 2559 (2006).
dotted lines represent the 1
13. J. F. Price, T. B. Sanford, G. Z. Forristall, J. Phys.
window for CD reported in Oceanogr. 24, 233 (1994).
(6), whereas the blue dots 14. Materials and methods are available as supporting
represent CD reported in (4). material on Science Online.
15. G. T. Mitchum, W. Sturges, J. Phys. Oceanogr. 12, 1310
0 (1982).
20 25 30 35 40 45 50 55
−1 16. S. T. Lentz, J. Phys. Oceanogr. 24, 2461 (1994).
Wind Speed (W , m s )
10 17. S. J. Lentz, J. Phys. Oceanogr. 31, 2749 (2001).
18. J. M. Pringle, J. Phys. Oceanogr. 32, 3101 (2002).
Downloaded from http://science.sciencemag.org/ on April 11, 2018
19. E. L. Andreas, J. Phys. Oceanogr. 34, 1429 (2004).
speeds below 30 m s−1, are somewhat noisy as a then steadily decreases as the wind speed 20. We thank M. S. Hulbert, A. J. Quaid, and W. A. Goode
for mooring support. We also thank the crews of the
result of measurement uncertainty and the need continues to rise. Our values for CD are in a research vessels Seward Johnson I and II. This work was
to calculate a velocity derivative, which tends to range of CD values found using meteorological supported by the Office of Naval Research as a part of
enhance noise. However, they consistently observations (4) for wind speeds greater than the Naval Research Laboratory’s basic research project
show a decreasing trend of CD for wind speeds 32 m s−1 but are higher for lower wind speeds. “Slope to Shelf Energetics and Exchange Dynamics
greater than 32 m s−1, the lower threshold for a These differences may be attributed to uncertain- (SEED)” under program element 0601153N, through the
Minerals Management Service Environmental Studies
category 1 hurricane on the Saffir-Simpson Scale. ties in the wind measurements and the applica- Program Technology, and by the Minerals Management
It is also apparent that the CD values are weakly bility of the simplified ocean dynamics at the Service Technology Assessment and Research Program on
dependent on the choice of the resistance co- lower wind speeds. Hurricane Ivan.
efficient and are larger for increasing values Supporting Online Material
of r. The drag coefficient estimates evaluated References and Notes www.sciencemag.org/cgi/content/full/315/5819/1707/DC1
for r = 0.1 cm s−1 are, on average, 20% greater 1. K. Emanuel, Nature 436, 686 (2005). SOM Text
than those calculated for r = 0.001 cm s−1 from 2. S. E. Larsen et al., in Wind Stress Over the Ocean, Fig. S1
I. S. F. Jones,Y. Toba, Eds. (Cambridge Univ. Press, References
Eq. 3. New York, 2001), chap. 7.
To produce the best representation of CD for 3. M. A. Donelan et al., Geophys. Res. Lett. 31, L18306 18 October 2006; accepted 14 February 2007
each r, a second-order curve (a function of the 10.1029/2004GL019460 (2004). 10.1126/science.1136466
wind speed) was fitted by a least-squares
technique to all estimated values of CD. The
curves are displayed in Figs. 2 and 3. Addition-
ally, the 95% confidence limits for the fitted
curve are shown in Fig. 3. The pattern of the
CRISPR Provides Acquired Resistance
relationship between CD and the wind speed is
robust, but the curve coefficients are determined
Against Viruses in Prokaryotes
by the value chosen for r in Eq. 3. However, all
Rodolphe Barrangou,1 Christophe Fremaux,2 Hélène Deveau,3 Melissa Richards,1
curves clearly show an initial increase of the drag
Patrick Boyaval,2 Sylvain Moineau,3 Dennis A. Romero,1 Philippe Horvath2*
coefficient and monotonic decrease as found by
recent studies (3–8) after reaching a maximum
Clustered regularly interspaced short palindromic repeats (CRISPR) are a distinctive feature of the
value at ~32 m s−1. Some of these studies (3, 19)
genomes of most Bacteria and Archaea and are thought to be involved in resistance to bacteriophages.
imply that the decreasing drag at high winds
We found that, after viral challenge, bacteria integrated new spacers derived from phage genomic
seems to be related to the spray, foam, and
sequences. Removal or addition of particular spacers modified the phage-resistance phenotype of the
bubbles from breaking waves that reduce the
cell. Thus, CRISPR, together with associated cas genes, provided resistance against phages, and
drag and allow the hurricane to slip over the sea.
resistance specificity is determined by spacer-phage sequence similarity.
With the nearly full water-column ocean cur-
rent measurements, the only unknown term left
in the simplified equation of motion is the wind acteriophages are arguably the most injection, restricting the incoming DNA, and
stress. Thus, the behavior of the drag coefficient
(CD) can easily be estimated for a range of strong
winds. Despite the fact that the drag coefficient is
B abundant biological entity on the planet
(1). Their ubiquitous distribution and
abundance have an important impact on micro-
abortive infection systems. These antiviral bar-
riers can also be engineered and manipulated to
better control phage populations (2, 3).
evaluated differently here, estimates of CD bial ecology and the evolution of bacterial Numerous bacteria have been selected by
determined “bottom-up” reasonably replicate genomes (2). Consequently, bacteria have devel- humans and used extensively for fermentation
the values determined “top-down” in recent oped a variety of natural defense mechanisms and biotechnology processes. Unfortunately, do-
studies (3–7). Results from our research show that target diverse steps of the phage life cycle, mesticated bacteria used in industrial applications
that CD peaks at a wind speed near 32 m s−1 and notably blocking adsorption, preventing DNA are often susceptible to phage attack, including
www.sciencemag.org SCIENCE VOL 315 23 MARCH 2007 1709
REPORTS
genera and species widely used as dairy cultures Nine phage-resistant mutants were generated in the CRISPR1 locus of the various phage-
(4). Accordingly, the industry has devised various independently by challenging the WT strain with resistant mutants revealed similarity to sequences
strategies to combat phage based on strain di- phage 858, phage 2972, or simultaneously with found within the genomes of the phages used in
versity, bacteriophage-insensitive mutants, and both (12), and their CRISPR loci were analyzed. the challenge (Fig. 2 and fig. S2). Interestingly,
plasmids bearing phage-resistance mechanisms. Differences were consistently observed at the similarities were observed throughout the phage
Streptococcus thermophilus is a low G+C CRISPR1 locus, where 1 to 4 additional spacers genomes, in most functional modules, both on the
Gram-positive bacterium and a key species ex- were inserted next to the 32 spacers present in coding and noncoding strands. No particular se-
ploited in the formulation of dairy culture sys- the WT strain (Fig. 1). The addition of new quence, gene, or functional group seemed to be
tems for the production of yogurt and cheese. spacers in response to phage infection seemed to targeted specifically. These results reveal that, on
Comparative genomics analyses of closely be polarized toward one end of the CRISPR1 lo- becoming resistant to bacteriophages, the CRISPR1
related S. thermophilus strains have previously cus. This is consistent with previous observations locus was modified by the integration of novel
revealed that genetic polymorphism primarily of spacer hypervariability at the leader end of the spacers, apparently derived from phage DNA.
occurs at hypervariable loci, such as the eps and CRISPR locus in various strains (9, 13). Se- Surprisingly, we observed that some strains
rps operons, as well as two clustered regularly quence analysis of the additional spacers inserted were resistant to both phages, whereas others
interspaced short palindromic repeats (CRISPR)
loci (5–7). CRISPR loci typically consist of sev-
eral noncontiguous direct repeats separated by cas5 cas1 cas6 cas7 repeat/spacer region ORF
stretches of variable sequences called spacers and
are oftentimes adjacent to cas genes (CRISPR- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32
L
associated). Although the function of CRISPR T
loci has not been established biologically, in
silico analyses of the spacers have revealed se- Sensitivity to Φ858 Sensitivity to Φ2972
Downloaded from http://science.sciencemag.org/ on April 11, 2018
10-7 10-6 10-5 10-4 10-3 10-2 10-1 1 10-7 10-6 10-5 10-4 10-3 10-2 10-1 1
quence homology with foreign elements, includ-
ing bacteriophage and plasmid sequences (7–9). L 1 2 WT
Based exclusively on in silico analyses, several
S1
S2
L 1 2 WTΦ858+S1S2
hypotheses have been put forward proposing
roles for CRISPR and cas genes, which include
S3
L 1 2 WTΦ858+S3
providing immunity against foreign genetic ele-
S4
ments via a mechanism based on RNA inter- L 1 2 WTΦ2972+S4
ference (10).
S5
L 1 2 WTΦ2972+S5
We analyzed the CRISPR sequences of vari-
S6
ous S. thermophilus strains, including closely L 1 2 WTΦ2972+S6
related industrial strains and phage-resistant var-
S7
L 1 2 WTΦ2972+S7
iants (fig. S1). Differences in the number and
type of spacers were observed primarily at the
S8
L 1 2 WTΦ2972+S8
CRISPR1 locus. Notably, phage sensitivity ap-
S11
S10
S12
S9
peared to be correlated with CRISPR1 spacer L 1 2 WTΦ858Φ2972+S9S10S11S12
content. Specifically, spacer content was nearly
S13
S14
L 1 2 WTΦ858Φ2972+S13S14
identical between parental strains and phage-
resistant derivatives, except for additional spacers Fig. 1. Streptococcus thermophilus CRISPR1 locus overview, newly acquired spacers in phage-
present in the latter. These findings therefore resistant mutants, and corresponding phage sensitivity. The CRISPR1 locus of DGCC7710 (WT) is at
suggest a potential relation between the presence the top. The repeat-spacer region of WT is in the middle: repeats (black diamonds), spacers
of additional spacers and the differences ob- (numbered gray boxes), leader (L, white box), and terminal repeat (T, black diamond). (Bottom left)
served in the phage sensitivity of a given strain. The spacer content on the leader side of the locus in phage-resistant mutants is detailed, with
This observation prompted us to investigate the newly acquired spacers (white boxes, S1 to S14). (Bottom right) The sensitivity of each strain to
origin and function of additional spacers present phages 858 and 2972 is represented as a histogram of the efficiency of plaquing (EOP), which is
in phage-resistant mutants. the plaque count ratio of a mutant strain to that of the wild-type.
First, we tested the hypothesis that CRISPR
loci are altered during the natural generation S9 S11 S3 S12 S5* S1 S4* S13
of phage-resistant mutants. A phage-host model
system was selected, consisting of a phage- Φ858 12 3 4 5 6 7 8 9 10 11
12131415161718 19 20 21 22 23225
426
272829303132
33435
3 36 37 38 39 40 4142
43444546
sensitive wild-type S. thermophilus strain widely S14 S7 S10 S2* S8* S6
used in the dairy industry, DGCC7710 [wild capsid host transcription
packaging tail morphogenesis replication
type (WT)] and two distinct but closely related morphogenesis lysis regulation
virulent bacteriophages isolated from industrial S9 S11 S3 S12 S5 S1* S4 S13
yogurt samples, phage 858 and phage 2972 (11).
Φ2972 1 2 3 4 5 6 7 8 9 10
11121314151617 18 19 20 21 2222
34252627
28
293033233
1 34 35 36 37 38 39441
0 424344
1
Danisco USA Inc., 3329 Agriculture Drive, Madison, WI S14 S7 S10 S2* S8 S6
1 kb
53716, USA. 2Danisco France SAS, Boîte Postale 10, F-86220
Dangé-Saint-Romain, France. 3Département de Biochimie et
Fig. 2. S. thermophilus phage genome maps with the position of sequences similar to the acquired
de Microbiologie, Faculté des Sciences et de Génie, Groupe
de Recherche en Ecologie Buccale, Faculté de Médecine CRISPR1 spacers of the phage-resistant mutants. Spacers shown above and below the genome maps
Dentaire, Félix d’Hérelle Reference Center for Bacterial indicate that the spacer matches a sequence on the (+) and on the (–) strand, respectively. An
Viruses, Université Laval, G1K 7P4 Québec, Canada. asterisk indicates the existence of a SNP between the spacer sequence and that of the phage
*To whom correspondence should be addressed. E-mail: genome (fig. S1). The genome sequences of phage 2972 (accession number AY699705) and phage
philippe.horvath@danisco.com 858 are 93% identical.
1710 23 MARCH 2007 VOL 315 SCIENCE www.sciencemag.org
REPORTS
were resistant only to the phage used in the chal- To determine whether CRISPR spacer con- these observed modifications establish the link
lenge (Fig. 1). The phage-resistance profile tent defines phage resistance, we altered the between the CRISPR spacer content and phage
seemed correlated to the spacer content, such CRISPR1 locus by adding and deleting spacers resistance.
that strains with spacers showing 100% identity (12) and tested subsequent strain sensitivity to In the process of generating strain
to sequences conserved in both phages were phages. All constructs were generated and inte- W T Ф 8 5 8 + S 1 S 2 ∆CRISPR1, we created
resistant to both phages, such as spacers S3, S6, grated into the S. thermophilus chromosome with WTФ858+S1S2::pR, a variant that contains the inte-
and S7. In contrast, when nucleotide polymor- the system developed by Russell and Klaenhammer gration vector with a single repeat inserted be-
phisms were observed between the spacer and the (14). We removed the spacers and repeats in the tween the cas genes and the native CRISPR1 locus
phage sequence [from 1 to 15 single-nucleotide CRISPR1 locus of strain WTФ858+S1S2 and replaced (Fig. 3). Unexpectedly, strain WTФ858+S1S2::pR
polymorphisms (SNPs) over 29 or 30 nucleo- them with a single repeat without any spacer (12). was sensitive to phage 858, although spacers
tides], the spacer did not seem to provide re- The resulting strain WTФ858+S1S2∆CRISPR1 was S1 and S2 remained on the chromosome (Fig. 3).
sistance, such as spacers S1, S2, S4, S5, and S8 sensitive to phage 858, which indicated that the Similarly, the WTФ2972+S4::pS1S2 construct lost
(Fig. 1 and fig. S2). In addition, when several phage resistance of the original phage-resistant the resistance to phage 2972, although spacer
spacers were inserted (S9 to S14), phage re- mutant (WTФ858+S1S2) was probably linked to S4 is present in the chromosome (Fig. 3). These
sistance levels were higher. These findings indi- the presence of S1 and S2 (Fig. 3). results indicated that spacers alone did not
cate that the CRISPR1 locus is subject to dynamic Further, to address the critical question of provide resistance, and perhaps, that they have
and rapid evolutionary changes driven by phage whether adding spacers provides novel phage to be in a particular genetic context to be
exposure. Altogether, these results reveal that resistance, we replaced the CRISPR1 locus of effective.
CRISPR loci can indeed be altered during the strain WTФ2972+S4 with a version containing only Although initial work suggested involvement
generation of phage-resistant mutants and also spacers S1 and S2 (12) and tested whether the in DNA repair (15), the current hypothesis is that
establish a link between CRISPR content and phage sensitivity was affected. Remarkably, the cas genes (5, 16) are involved in CRISPR-
phage sensitivity. These findings suggest that the resulting strain WTФ2972+S4::pS1S2 gained re- mediated immunity (10). Consequently, we in-
Downloaded from http://science.sciencemag.org/ on April 11, 2018
presence of a CRISPR spacer identical to a phage sistance to phage 858, which suggested that activated two cas genes in strain WTФ858+S1S2
sequence provides resistance against phages these two spacers have the ability to provide (12): cas5 (COG3513) and cas7, which are equiv-
containing this particular sequence. phage resistance de novo (Fig. 3). Altogether, alent to str0657/stu0657 and str0660/stu0660,
respectively (6, 7). The cas5 inactivation re-
sulted in loss of the phage resistance (Fig. 3),
S2
and perhaps Cas5 acts as a nuclease, because it
S1
L 1 2
cas5 cas1 cas6 cas7 ORF
1 kb contains an HNH-type nuclease motif. In con-
I. trast, inactivating cas7 did not alter the resist-
L T ance to phage 858 (Fig. 3). Interestingly, we
cas5 cas1 cas6 cas7 ORF were repeatedly unable to generate CRISPR1
II. phage-resistant mutants from the cas7 knock-
out, perhaps because Cas7 is involved in the
S1
S2
L T L 1 2
synthesis and/or insertion of new spacers and
cas5 cas1 cas6 cas7 pORI ORF
III. additional repeats.
When we tested the sensitivity of the phage-
S1
S2
S4
L T L 1 2 resistant mutants, we found that plaque formation
cas5 cas1 cas6 cas7 pORI ORF was dramatically reduced, but that a relatively
IV.
small population of bacteriophage retained the
ability to infect the mutants. We further analyzed
S1
S2
L 1 2
pORI cas1 cas6 cas7 ORF phage variants derived from phage 858 that
V. retained the ability to infect WTФ858+S1S2. In par-
ticular, we investigated the sequence of the ge-
S2
S1
L 1 2
nome region corresponding to additional spacers
cas5 cas1 cas6 pORI ORF
VI. S1 and S2 in two virulent phage variants. In both
cases, the genome sequence of the phage var-
Sensitivity to Φ858 Sensitivity to Φ2972 iant had mutated, and two distinct SNPs were
10-7 10-6 10-5 10-4 10-3 10-2 10-1 1 10-7 10-6 10-5 10-4 10-3 10-2 10-1 1
identified in the sequence corresponding to
I. WTΦ858+S1S2 spacer S1 (fig. S3).
II. WTΦ858+S1S2∆CRISPR1 Overall, prokaryotes appear to have evolved
a nucleic acid–based “immunity” system where-
III. WTΦ858+S1S2::pR
by specificity is dictated by the CRISPR spacer
IV. WTΦ2972+S4::pS1S2 content, while the resistance is provided by the
V. WTΦ858+S1S2::pcas5– Cas enzymatic machinery. Additionally, we spec-
ulate that some of the cas genes not directly
VI. WTΦ858+S1S2::pcas7–
providing resistance are actually involved in the
Fig. 3. CRISPR spacer engineering, cas gene inactivation, and corresponding phage sensi- insertion of additional CRISPR spacers and re-
tivity. I, mutant WT F858+S1S2; II, mutant WT F858+S1S2DCRISPR1 in which CRISPR1 was deleted; peats, as part of an adaptive “immune” response.
III, mutant WTF858+S1S2::pR in which CRISPR1 was displaced and replaced with a unique repeat; Further studies are desired to better characterize
IV, WTF2972+S4::pS1S2, mutant of strain WTF2972+S4 in which CRISPR1 was displaced and re- the mechanism of action and to identify the
placed with a version containing S1 and S2; V, WTF858+S1S2::pcas5– with cas5 inactivated; VI, specific function of the various cas genes. This
WTF858+S1S2::pcas7– with cas7 inactivated. pORI indicates the integrated plasmid (12). The phage nucleic acid–based system contrasts with amino
sensitivity of each strain to phages 858 and 2972 is represented at the bottom as a histogram of acid–based counterparts in eukaryotes through
the efficiency of plaquing (EOP). which adaptative immunity is not inheritable.
www.sciencemag.org SCIENCE VOL 315 23 MARCH 2007 1711
REPORTS
The inheritable nature of CRISPR spacers sup- References and Notes 16. D. H. Haft, J. Selengut, E. F. Mongodin, K. E. Nelson,
ports the use of CRISPR loci as targets for evo- 1. M. Breitbart, F. Rohwer, Trends Microbiol. 13, 278 PloS Comput. Biol. 1, e60 (2005).
(2005). 17. P. M. A. Groenen, A. E. Bunschoten, D. van Soolingen,
lutionary, typing, and comparative genomic studies 2. S. Chibani-Chennoufi, A. Bruttin, M.-L. Dillmann, H. Brüssow, J. D. A. van Embden, Mol. Microbiol. 10, 1057 (1993).
(9, 17–19). Because this system is reactive to J. Bacteriol. 186, 3677 (2004). 18. E. F. Mongodin et al., J. Bacteriol. 187, 4935 (2005).
the phage environment, it likely plays a sig- 3. J. M. Sturino, T. R. Klaenhammer, Nat. Rev. Microbiol. 4, 19. R. T. DeBoy, E. F. Mongodin, J. B. Emerson, K. E. Nelson,
nificant role in prokaryotic evolution and ecol- 395 (2006). J. Bacteriol. 188, 2364 (2006).
4. H. Brüssow, Annu. Rev. Microbiol. 55, 283 (2001). 20. R. W. Hendrix et al., Proc. Natl. Acad. Sci. U.S.A. 96,
ogy and provides a historical perspective of 5. R. Jansen, J. D. A. van Embden, W. Gaastra, L. M. Schouls, 2192 (1999).
phage exposure, as well as a predictive tool for Mol. Microbiol. 43, 1565 (2002). 21. J. S. Godde, A. Bickerton, J. Mol. Evol. 62, 718
phage sensitivity. The CRISPR-cas system may 6. A. Bolotin et al., Nat. Biotechnol. 22, 1554 (2004). (2006).
7. A. Bolotin, B. Quinquis, A. Sorokin, S. D. Ehrlich, 22. We thank L. Bayer, C. Vos, and A.-C. Coûté-Monvoisin
accordingly be exploited as a virus defense mech- Microbiology 151, 2551 (2005). of Danisco Innovation, as well as J. Labonté and
anism and also potentially used to reduce the 8. F. J. M. Mojica, C. Díez-Villaseñor, J. García-Martínez, D. Tremblay of Université Laval for technical support, and
dissemination of mobile genetic elements and E. Soria, J. Mol. Evol. 60, 174 (2005). E. Bech Hansen for discussions and critical review of
the acquisition of undesirable traits such as anti- 9. C. Pourcel, G. Salvignol, G. Vergnaud, Microbiology 151, the manuscript. Also, we thank T. R. Klaenhammer for
biotic resistance genes and virulence markers. 653 (2005). providing the integration system. This work was
10. K. S. Makarova, N. V. Grishin, S. A. Shabalina, Y. I. Wolf, supported by funding from Danisco A/S. Also, S. M. would
From a phage evolution perspective, the inte- E. V. Koonin, Biol. Direct 1, 7 (2006). like to acknowledge support from the Natural Sciences
grated phage sequences within CRISPR loci may 11. C. Lévesque et al., Appl. Environ. Microbiol. 71, 4057 and Engineering Research Council of Canada (NSERC)
also provide additional anchor points to facilitate (2005). Discovery Program. Sequences were deposited in
recombination during subsequent phage infec- 12. Information on materials and methods for the generation GenBank, accession numbers EF434458 to EF434504.
of phage-resistant mutants, engineering of CRISPR
tions, thus increasing the gene pool to which spacers (Figs. S4 and S5), and inactivation of cas genes is Supporting Online Material
phages have access (20). Because CRISPR loci available on Science Online. www.sciencemag.org/cgi/content/full/315/5819/1709/DC1
are found in the majority of bacterial genera and 13. R. K Lillestøl, P. Redder, R. A. Garrett, K. Brügger, Materials and Methods
are ubiquitous in Archaea (5, 13, 21), their study Archaea 2, 59 (2006). Figs. S1 to S5
Downloaded from http://science.sciencemag.org/ on April 11, 2018
14. W. M. Russell, T. R. Klaenhammer, Appl. Environ. Microbiol. References and Notes
will provide new insights into the relation and 67, 4361 (2001).
codirected evolution between prokaryotes and 15. K. S. Makarova, L. Aravind, N. V. Grishin, I. B. Rogozin, 29 November 2006; accepted 16 February 2007
their predators. E. V. Koonin, Nucleic Acids Res. 30, 482 (2002). 10.1126/science.1138140
A G Protein–Coupled Receptor Is a Arabidopsis putative GPCR protein (GCR1) has
been characterized in plants (17–20), and no
ligand has been defined for any plant GPCR.
Plasma Membrane Receptor for the To identify previously unrecognized GPCR
proteins in Arabidopsis, we started by searching
Plant Hormone Abscisic Acid the Arabidopsis genome and found a gene
(GCR2, GenBank accession code At1g52920)
encoding a putative GPCR. Transmembrane
Xigang Liu,1,2 Yanling Yue,1 Bin Li,3 Yanli Nie,1 Wei Li,2 Wei-Hua Wu,3 Ligeng Ma1,2* structure prediction suggests that GCR2 is a
membrane protein with seven transmembrane
The plant hormone abscisic acid (ABA) regulates many physiological and developmental processes helices (fig. S1, A and B). The subsequent cel-
in plants. The mechanism of ABA perception at the cell surface is not understood. Here, we lular localization analysis confirmed its plasma
report that a G protein–coupled receptor genetically and physically interacts with the G protein membrane localization in the transgenic plant
a subunit GPA1 to mediate all known ABA responses in Arabidopsis. Overexpressing this receptor root (fig. S1C). GCR2–yellow fluorescent pro-
results in an ABA-hypersensitive phenotype. This receptor binds ABA with high affinity at tein (YFP) is detected in the membrane fraction
physiological concentration with expected kinetics and stereospecificity. The binding of ABA to the isolated from the GCR2-YFP transgenic plant.
receptor leads to the dissociation of the receptor-GPA1 complex in yeast. Our results demonstrate Similar to GCR1 (19), GCR2 is mostly asso-
that this G protein–coupled receptor is a plasma membrane ABA receptor. ciated with the membrane fraction (fig. S1D).
Furthermore, even after washing with detergent
bscisic acid (ABA) is an important In contrast, several earlier experiments had sug- or a higher pH buffer, GCR2 is retained with the
A hormone that mediates many aspects of
plant growth and development, particu-
larly in response to the environmental stresses
gested that extracellular perception is critical for
ABA to achieve its functions (7–9). Thus, other
ABA receptors, especially plasma membrane–
membrane fraction, suggesting that GCR2 is an
integral membrane protein (fig. S1D).
One feature of the GPCR is its ability to
(1–3). Several components involved in the ABA localized receptors, may be the major players for interact with G protein to form a complex. To
signaling pathway have been identified (4). Two perceiving extracellular ABA and mediating the confirm the physical interaction between GCR2
recent reports have shown that the nuclear RNA classic ABA signaling responses. and Ga, we used four different approaches to
binding protein flowering time control protein Ligand-mediated signaling through G protein– detect their interaction. We first used surface
(FCA) (5) and the chloroplast protein Mg coupled receptors (GPCRs) is a conserved plasmon resonance spectroscopy to investigate
chelatase H subunit (6) are ABA receptors (6). mechanism for the extracellular signal percep- the interaction between GCR2 and GPA1. For
tion at the plasma membrane in eukaryotic this purpose, we expressed and purified recom-
1
National Institute of Biological Sciences, 7 Science Park Road, organisms (10). The GPCR-mediated signaling binant GCR2 and GPA1 proteins in bacteria
Zhongguancun Life Science Park, Beijing 102206, China. pathway plays a central role in vital processes (fig. S2). This in vitro assay clearly indicated
2
Laboratory of Molecular and Cellular Biology, Hebei Normal such as vision, taste, and olfaction in animals that GPA1 is capable of binding to GCR2, where-
University, Shijiazhuang, Hebei 050016, China. 3State Key (11). However, the higher plant Arabidopsis as no binding activity was detected between
Laboratory of Plant Physiology and Biochemistry, College of
Biological Sciences, China Agricultural University, Beijing thaliana has only one canonical Ga (GPA1) GPA1 and bovine serum albumin (BSA) (fig.
100094, China. subunit, one Gb subunit, and two Gg subunits S3, A and B). The dissociation binding con-
*To whom correspondence should be addressed. E-mail: (12–16). The significance of these subunits in stant (Kd) for GCR2 and GPA1 is 2.1 × 10−9 M
maligeng@nibs.ac.cn plant systems is poorly understood; only one (fig. S3C).
1712 23 MARCH 2007 VOL 315 SCIENCE www.sciencemag.org
CRISPR Provides Acquired Resistance Against Viruses in Prokaryotes
Rodolphe Barrangou, Christophe Fremaux, Hélène Deveau, Melissa Richards, Patrick Boyaval, Sylvain Moineau, Dennis A.
Romero and Philippe Horvath
Science 315 (5819), 1709-1712.
DOI: 10.1126/science.1138140
Downloaded from http://science.sciencemag.org/ on April 11, 2018
ARTICLE TOOLS http://science.sciencemag.org/content/315/5819/1709
SUPPLEMENTARY http://science.sciencemag.org/content/suppl/2007/03/19/315.5819.1709.DC1
MATERIALS
RELATED http://science.sciencemag.org/content/sci/315/5819/1650.1.full
CONTENT
REFERENCES This article cites 20 articles, 6 of which you can access for free
http://science.sciencemag.org/content/315/5819/1709#BIBL
PERMISSIONS http://www.sciencemag.org/help/reprints-and-permissions
Use of this article is subject to the Terms of Service
Science (print ISSN 0036-8075; online ISSN 1095-9203) is published by the American Association for the Advancement of
Science, 1200 New York Avenue NW, Washington, DC 20005. 2017 © The Authors, some rights reserved; exclusive
licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. The title
Science is a registered trademark of AAAS.
You might also like
- Devlin Biochemistry With Clinical CorrelationsDocument1,241 pagesDevlin Biochemistry With Clinical CorrelationsJorge100% (5)
- Summary of Georgia Immunization Requirements For Child Care & School AttendanceDocument1 pageSummary of Georgia Immunization Requirements For Child Care & School AttendanceWSB-TV Assignment DeskNo ratings yet
- 3753 Full PDFDocument3 pages3753 Full PDFJames WatsonNo ratings yet
- Foundations in Microbiology Basic Principles 10th Edition Talaro Test BankDocument35 pagesFoundations in Microbiology Basic Principles 10th Edition Talaro Test Bankhieudermotjm7w100% (28)
- Paper I PDFDocument6 pagesPaper I PDFErick FariasNo ratings yet
- Benazet El Al. Science (2009) A Self-Regulatory System of Interlinked SignalingDocument5 pagesBenazet El Al. Science (2009) A Self-Regulatory System of Interlinked SignalingnovrodNo ratings yet
- Science 1213229Document5 pagesScience 12132291592162022No ratings yet
- Ultrahigh Porosity in Metal-Organic Frameworks: 'KeeffeDocument6 pagesUltrahigh Porosity in Metal-Organic Frameworks: 'Keeffeクマール ヴァンツNo ratings yet
- Singer Et Al2004Document6 pagesSinger Et Al2004Firuza RahimovaNo ratings yet
- A Physical Map of 30,000 Human Genes: J. Am. Chem. Soc. 117, 4193 (1995) ), As DemonstratDocument3 pagesA Physical Map of 30,000 Human Genes: J. Am. Chem. Soc. 117, 4193 (1995) ), As DemonstratRahmat Eko SanjayaNo ratings yet
- Gregg 2010Document5 pagesGregg 2010Daniel Nuno Vancetto BorgesNo ratings yet
- PWYW1Document3 pagesPWYW1Andrew GarfieldNo ratings yet
- Boletin de Arquelologia PUCP No. 16 (2014) Número 16. Los Rostros de Huari Perspectivas Interregionales Sobre El Horizonte MedioDocument5 pagesBoletin de Arquelologia PUCP No. 16 (2014) Número 16. Los Rostros de Huari Perspectivas Interregionales Sobre El Horizonte MedioPareja Anyosa DanteNo ratings yet
- Judy MikovitsDocument7 pagesJudy MikovitsJ Elver SilvaNo ratings yet
- PankjoyDocument2 pagesPankjoyMonitchelle Cristina do NascimentoNo ratings yet
- Nuclei Get TAN LinesDocument3 pagesNuclei Get TAN LinesArpan NandyNo ratings yet
- Excitation of Orbital Angular Momentum Resonances in Helically Twisted Photonic Crystal FiberDocument4 pagesExcitation of Orbital Angular Momentum Resonances in Helically Twisted Photonic Crystal Fiberthillai saravananNo ratings yet
- Magnetic Skyrmions: Advances in Physics and Potential ApplicationsDocument5 pagesMagnetic Skyrmions: Advances in Physics and Potential ApplicationsAgtc TandayNo ratings yet
- Reconstructing signaling networks from single-cell dataDocument8 pagesReconstructing signaling networks from single-cell dataarchsarkNo ratings yet
- E.Behnke Et Al., Science, 319, (2008) - Spin Dependent WIMP Limits From A Bubble ChamberDocument4 pagesE.Behnke Et Al., Science, 319, (2008) - Spin Dependent WIMP Limits From A Bubble ChamberIvan FelisNo ratings yet
- 4D Electron Tomography ScienceDocument6 pages4D Electron Tomography Sciencehappynewyear11No ratings yet
- Teleport Nonclassical WavepacklightDocument4 pagesTeleport Nonclassical Wavepacklighth tytionNo ratings yet
- Simulating Dynamical Features of Escape Panic: Dirk Helbing, Illeâs Farkas & Tamaâs VicsekDocument4 pagesSimulating Dynamical Features of Escape Panic: Dirk Helbing, Illeâs Farkas & Tamaâs VicsektariqNo ratings yet
- OSTROM, Elinor. A General Framework For Analizing Sustaintability of Socio Ecological SystemsDocument5 pagesOSTROM, Elinor. A General Framework For Analizing Sustaintability of Socio Ecological SystemsLizbeth Diaz RedolfoNo ratings yet
- Parrenin Science 2013Document5 pagesParrenin Science 2013Camilo CastellanosNo ratings yet
- Quantom Dot Induced Phase Stabilization of the Cspbi3 Perovskite for High Efficiency PhotovoltaicsDocument5 pagesQuantom Dot Induced Phase Stabilization of the Cspbi3 Perovskite for High Efficiency PhotovoltaicsabuhurairabscNo ratings yet
- The Following Resources Related To This Article Are Available Online atDocument5 pagesThe Following Resources Related To This Article Are Available Online atihzaoloanNo ratings yet
- Active Normal Faulting in The Upper Rhine Graben and Paleoseismic Identification of The 1356 Basel Earthquake.Document5 pagesActive Normal Faulting in The Upper Rhine Graben and Paleoseismic Identification of The 1356 Basel Earthquake.marceloahumada1983No ratings yet
- 5-2013-Research-Multiplex Genome Engineering Using CRISPRCas SystemsDocument6 pages5-2013-Research-Multiplex Genome Engineering Using CRISPRCas SystemsCristian Felipe Sandoval QuiñonezNo ratings yet
- Ross 2013Document5 pagesRoss 2013zune153No ratings yet
- T. Rex Was A Slow RunnerDocument4 pagesT. Rex Was A Slow RunnerozzaibNo ratings yet
- Barrangou 20 Et 20 Al 202007Document6 pagesBarrangou 20 Et 20 Al 202007ירדן לויןNo ratings yet
- In Modern Shells Southern Nevada: Taylor AstrophysDocument4 pagesIn Modern Shells Southern Nevada: Taylor AstrophysVitaliy T.No ratings yet
- Suppression of Rain and Snow by Urban and Industrial Air PollutionDocument6 pagesSuppression of Rain and Snow by Urban and Industrial Air PollutionDavidNo ratings yet
- Science (New York NY) 2003 TemelesDocument4 pagesScience (New York NY) 2003 TemelesmariaNo ratings yet
- Articulo Cientico 2Document5 pagesArticulo Cientico 2Elvis AndíaNo ratings yet
- A Logic-Gated Nanorobot For Targeted Transport of Molecular PayloadsDocument5 pagesA Logic-Gated Nanorobot For Targeted Transport of Molecular PayloadsJohnathonNo ratings yet
- Chen and Tung 2014 ScienceDocument8 pagesChen and Tung 2014 Science1739598671No ratings yet
- Acfrogcfqtzu2xnftwqjnegs7ygzf1guo4 K Hves Qm5urgs6j7bracmbcs4px1djm2jtjpktog2kwjhdexqbirxrvocnrlkvl6dixeprgtv0xlrmwujhdy5qi6md71yupr4hwrgyj9ym1ev A6Document6 pagesAcfrogcfqtzu2xnftwqjnegs7ygzf1guo4 K Hves Qm5urgs6j7bracmbcs4px1djm2jtjpktog2kwjhdexqbirxrvocnrlkvl6dixeprgtv0xlrmwujhdy5qi6md71yupr4hwrgyj9ym1ev A6Aniket AmanNo ratings yet
- 1041 FullDocument6 pages1041 FullAdriano ItoNo ratings yet
- 11.ORoak Et Al 2012Document77 pages11.ORoak Et Al 2012MiaNo ratings yet
- 2016-Sung-Jin Park-Phototactic guidance of a tissue-engineered soft-robotic rayDocument6 pages2016-Sung-Jin Park-Phototactic guidance of a tissue-engineered soft-robotic rayitm.ggwpNo ratings yet
- Role of Genotype in The Cycle of Violence in Maltreated ChildrenDocument5 pagesRole of Genotype in The Cycle of Violence in Maltreated ChildrenMiquel AlaberniaNo ratings yet
- A General Framework For Analyzing Sustainability of Social-Ecological SystemsDocument5 pagesA General Framework For Analyzing Sustainability of Social-Ecological SystemsgranaditoshshNo ratings yet
- A General Framework For Analyzing Sustainability of Social-Ecological SystemsDocument4 pagesA General Framework For Analyzing Sustainability of Social-Ecological SystemsAlessandro Roberto de OliveiraNo ratings yet
- Iceland Fertility CousinsDocument5 pagesIceland Fertility CousinshalfdeadNo ratings yet
- Nature NeuropsicologiaDocument3 pagesNature NeuropsicologiaGabrielVásquezNo ratings yet
- Influence of Life Stress OnDocument4 pagesInfluence of Life Stress Ondeiab842728No ratings yet
- He Cken Berger 92008Document6 pagesHe Cken Berger 92008llerenasNo ratings yet
- Barriopedro 11Document6 pagesBarriopedro 11JxPNo ratings yet
- The Following Resources Related To This Article Are Available Online atDocument6 pagesThe Following Resources Related To This Article Are Available Online atChia-Lin Charles HoNo ratings yet
- Science 1201080Document5 pagesScience 1201080NeerajNo ratings yet
- Reports: Experimental Realization of Wheeler's Delayed-Choice Gedanken ExperimentDocument4 pagesReports: Experimental Realization of Wheeler's Delayed-Choice Gedanken ExperimentreflectiondarkNo ratings yet
- D C EliasScience 323, 610 (2009)Document5 pagesD C EliasScience 323, 610 (2009)Tania ChatterjeeNo ratings yet
- 2009 Science Jimenez CompleteDocument26 pages2009 Science Jimenez CompleteDarko PopovskiNo ratings yet
- Spatiotemporal Microbial Evolution On Antibiotic Landscapes: 13 ReferencesandnotesDocument6 pagesSpatiotemporal Microbial Evolution On Antibiotic Landscapes: 13 ReferencesandnotesJaqueline Godinez CamachoNo ratings yet
- 60.full Photochemical ProcessDocument5 pages60.full Photochemical ProcessDhanashri Rathod 18357No ratings yet
- High-Throughput Synthesis of Zeolitic Imidazolate Frameworks and Application To CO2 Capture (DRX)Document6 pagesHigh-Throughput Synthesis of Zeolitic Imidazolate Frameworks and Application To CO2 Capture (DRX)Annaíres AlmeidaNo ratings yet
- Catalytic: Cracking Acetic Acid To AceticDocument6 pagesCatalytic: Cracking Acetic Acid To AceticPawan NagarNo ratings yet
- Banerjee 2008Document6 pagesBanerjee 2008paulaNo ratings yet
- 2002 Irmof-2 EdusolDocument5 pages2002 Irmof-2 EdusolAlejandra AwimbaweNo ratings yet
- Cuprate Spin-Charge Order Observed in Quasi-Particle SpectrumDocument5 pagesCuprate Spin-Charge Order Observed in Quasi-Particle SpectrumAlejandra AwimbaweNo ratings yet
- A Modified Double Loop EPR Technique For Detection of Sensitization in Cast Stainless SteelDocument3 pagesA Modified Double Loop EPR Technique For Detection of Sensitization in Cast Stainless Steelcuentas ricardoNo ratings yet
- The Earth's Middle AtmosphereFrom EverandThe Earth's Middle AtmosphereW. L. GroseNo ratings yet
- Abbas Et Al. - 2016 - Unusual Complication of Pituitary Macroadenoma A Case Report and ReviewDocument5 pagesAbbas Et Al. - 2016 - Unusual Complication of Pituitary Macroadenoma A Case Report and ReviewflashjetNo ratings yet
- Abbas, Al-Arini, Meshikhes - 2014 - Gastric Autonomic Nerve Tumour A Rare Gastric Tumour PDFDocument2 pagesAbbas, Al-Arini, Meshikhes - 2014 - Gastric Autonomic Nerve Tumour A Rare Gastric Tumour PDFflashjetNo ratings yet
- Abbas Et Al. - 2020 - Maneuvering The Management of A Rare Case of Primary Undifferentiated Cardiac Sarcoma PDFDocument5 pagesAbbas Et Al. - 2020 - Maneuvering The Management of A Rare Case of Primary Undifferentiated Cardiac Sarcoma PDFflashjetNo ratings yet
- Nampei Et Al. - 2020 - Comparison of Ocular Surface Squamous Neoplasia and Pterygium Using Anterior Segment Optical Coherence Tomography PDFDocument4 pagesNampei Et Al. - 2020 - Comparison of Ocular Surface Squamous Neoplasia and Pterygium Using Anterior Segment Optical Coherence Tomography PDFflashjetNo ratings yet
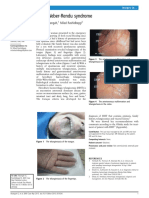
- Abangah, Rashidbeygi - 2013 - Osler-Weber-Rendu SyndromeDocument2 pagesAbangah, Rashidbeygi - 2013 - Osler-Weber-Rendu SyndromeflashjetNo ratings yet
- Nascimento Et Al. - 2013 - Wearable Cardioverter Defibrillator in Stress Cardiomyopathy and Cardiac ArrestDocument4 pagesNascimento Et Al. - 2013 - Wearable Cardioverter Defibrillator in Stress Cardiomyopathy and Cardiac ArrestflashjetNo ratings yet
- Nana Et Al. - 2014 - Left Renal Vein Thrombosis A Rare Cause of Acute Scrotal Pain PDFDocument2 pagesNana Et Al. - 2014 - Left Renal Vein Thrombosis A Rare Cause of Acute Scrotal Pain PDFflashjetNo ratings yet
- Abbas Et Al. - 2013 - Posterior Reversible Encephalopathy Syndrome After Bevacizumab Therapy in A Normotensive PatientDocument4 pagesAbbas Et Al. - 2013 - Posterior Reversible Encephalopathy Syndrome After Bevacizumab Therapy in A Normotensive PatientflashjetNo ratings yet
- Nascimento Et Al. - 2013 - Asymptomatic Anomalous Left Anterior Descending Artery Arising From The Right Coronary Artery With A Rare AntDocument3 pagesNascimento Et Al. - 2013 - Asymptomatic Anomalous Left Anterior Descending Artery Arising From The Right Coronary Artery With A Rare AntflashjetNo ratings yet
- Abbas Et Al. - 2016 - Unusual Complication of Pituitary Macroadenoma A Case Report and ReviewDocument5 pagesAbbas Et Al. - 2016 - Unusual Complication of Pituitary Macroadenoma A Case Report and ReviewflashjetNo ratings yet
- Namburar Et Al. - 2018 - Waste Generated During Glaucoma Surgery A Comparison of Two Global Facilities PDFDocument4 pagesNamburar Et Al. - 2018 - Waste Generated During Glaucoma Surgery A Comparison of Two Global Facilities PDFflashjetNo ratings yet
- Nathwani - 2013 - A Complicated Case of AnticoagulationDocument2 pagesNathwani - 2013 - A Complicated Case of AnticoagulationflashjetNo ratings yet
- Nascimento Et Al. - 2012 - Bloody Nipple Discharge in Infancy - Report of Two CasesDocument3 pagesNascimento Et Al. - 2012 - Bloody Nipple Discharge in Infancy - Report of Two CasesflashjetNo ratings yet
- Abbas Et Al. - 2013 - Posterior Reversible Encephalopathy Syndrome After Bevacizumab Therapy in A Normotensive PatientDocument4 pagesAbbas Et Al. - 2013 - Posterior Reversible Encephalopathy Syndrome After Bevacizumab Therapy in A Normotensive PatientflashjetNo ratings yet
- Narula, Nangia - 2014 - Use of An Oscillatory PEP Device To Enhance Bronchial Hygiene in A Patient of post-H1NI Pneumonia and Acute Resp PDFDocument3 pagesNarula, Nangia - 2014 - Use of An Oscillatory PEP Device To Enhance Bronchial Hygiene in A Patient of post-H1NI Pneumonia and Acute Resp PDFflashjetNo ratings yet
- Nasser Et Al. - 2013 - Use of Transoesophageal Echocardiography in Endovascular Stenting For Superior Vena Cava SyndromeDocument3 pagesNasser Et Al. - 2013 - Use of Transoesophageal Echocardiography in Endovascular Stenting For Superior Vena Cava SyndromeflashjetNo ratings yet
- Nascimento Et Al. - 2012 - Adrenoleukodystrophy A Forgotten Diagnosis in Children With Primary Addison' S DiseaseDocument5 pagesNascimento Et Al. - 2012 - Adrenoleukodystrophy A Forgotten Diagnosis in Children With Primary Addison' S DiseaseflashjetNo ratings yet
- Stroke and Transient Ischaemic Attack in Over 16s Diagnosis and Initial Management PDF 975574676437Document29 pagesStroke and Transient Ischaemic Attack in Over 16s Diagnosis and Initial Management PDF 975574676437MaryamNo ratings yet
- Nasrat, Al-Alwan - 2012 - Medical Intervention in A Constitutionally Tall ChildDocument3 pagesNasrat, Al-Alwan - 2012 - Medical Intervention in A Constitutionally Tall ChildflashjetNo ratings yet
- Nassani Et Al. - 2017 - Foreign Body Penetration Through Jejunal Loops Causing Renal Artery Thrombosis and Renal InfarctDocument3 pagesNassani Et Al. - 2017 - Foreign Body Penetration Through Jejunal Loops Causing Renal Artery Thrombosis and Renal InfarctflashjetNo ratings yet
- Idiopathic Stroke After Syndromic and Neuromuscular Scoliosis Surgery: A Case Report and Literature ReviewDocument7 pagesIdiopathic Stroke After Syndromic and Neuromuscular Scoliosis Surgery: A Case Report and Literature ReviewflashjetNo ratings yet
- Naveen Et Al. - 2014 - Alternatives For Restoration of A Hemisected Mandibular MolarDocument4 pagesNaveen Et Al. - 2014 - Alternatives For Restoration of A Hemisected Mandibular MolarflashjetNo ratings yet
- Natrella Et Al. - 2012 - Rare Disease Supratentorial Neurenteric Cyst Associated With A Intraparenchymal SubependymomaDocument6 pagesNatrella Et Al. - 2012 - Rare Disease Supratentorial Neurenteric Cyst Associated With A Intraparenchymal SubependymomaflashjetNo ratings yet
- Nassar, Abdul-Jawad, Barnes - 2018 - Hepatic Failure Due To Cholestatic Hepatitis C in An Immunosuppressed Patient Treated With ElbasvirDocument3 pagesNassar, Abdul-Jawad, Barnes - 2018 - Hepatic Failure Due To Cholestatic Hepatitis C in An Immunosuppressed Patient Treated With ElbasvirflashjetNo ratings yet
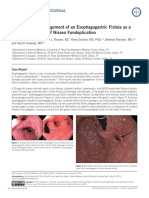
- Naveed Et Al. - 2015 - Diagnosis and Management of An Esophagogastric Fistula As A Rare Complication of Nissen FundoplicationDocument2 pagesNaveed Et Al. - 2015 - Diagnosis and Management of An Esophagogastric Fistula As A Rare Complication of Nissen FundoplicationflashjetNo ratings yet
- Nguyen Et Al. - 2020 - Human Leukocyte Antigen Susceptibility Map For Severe Acute Respiratory Syndrome Coronavirus 2Document36 pagesNguyen Et Al. - 2020 - Human Leukocyte Antigen Susceptibility Map For Severe Acute Respiratory Syndrome Coronavirus 2flashjetNo ratings yet
- Notes 200430 195226 0c2 PDFDocument19 pagesNotes 200430 195226 0c2 PDFflashjetNo ratings yet
- Notes 190915 213814 Ea7 PDFDocument1 pageNotes 190915 213814 Ea7 PDFflashjetNo ratings yet
- Naveen Et Al. - 2013 - Complete Denture With Pharyngeal Bulb ProsthesisDocument4 pagesNaveen Et Al. - 2013 - Complete Denture With Pharyngeal Bulb ProsthesisflashjetNo ratings yet
- Nguyen Et Al. - 2019 - Challenges in Management of Autoimmune Hepatitis With Concurrent Graves ThyrotoxicosisDocument4 pagesNguyen Et Al. - 2019 - Challenges in Management of Autoimmune Hepatitis With Concurrent Graves ThyrotoxicosisflashjetNo ratings yet
- Qualitative PPT 291118Document15 pagesQualitative PPT 291118Ixora MyNo ratings yet
- Essencial Natural Science Unit 1 TestDocument2 pagesEssencial Natural Science Unit 1 TestAlvaro Rizzo GonzálezNo ratings yet
- Sample exam questions on DNA, transcription, and translationDocument4 pagesSample exam questions on DNA, transcription, and translationLorraine M. Del RosarioNo ratings yet
- 1GS Cell PPT 2018Document73 pages1GS Cell PPT 2018pixiedustNo ratings yet
- The Cell Membrane: AP BiologyDocument53 pagesThe Cell Membrane: AP BiologythalesNo ratings yet
- Ashotinthearmora Hard Pill To Swallow?: Comparing Vaccine FormulationsDocument3 pagesAshotinthearmora Hard Pill To Swallow?: Comparing Vaccine FormulationsKaren SinorNo ratings yet
- Slip Test-5Document3 pagesSlip Test-5G.N. BhuvaneshwaranNo ratings yet
- Current Protocols in Molecular BiologyDocument11 pagesCurrent Protocols in Molecular BiologyMiriam Illescas0% (3)
- Fall 2021: Seminar 2: Cell Cycle, Mitosis and MeiosisDocument24 pagesFall 2021: Seminar 2: Cell Cycle, Mitosis and MeiosisChanceNo ratings yet
- Cell Structure and Function Test WorksheetDocument2 pagesCell Structure and Function Test WorksheetJohn Van Dave TaturoNo ratings yet
- Cell Cycle, mitosis worksheetDocument3 pagesCell Cycle, mitosis worksheetrosellevalerio2014No ratings yet
- Genomics Course: Basics of Genome SequencingDocument10 pagesGenomics Course: Basics of Genome Sequencingk.sachinNo ratings yet
- Types of MutationsDocument64 pagesTypes of MutationsI'm Cracked100% (1)
- MS-MS Analysis Programs - 2012 SlidesDocument14 pagesMS-MS Analysis Programs - 2012 SlidesJovanderson JacksonNo ratings yet
- Overview of Biochemical EndocrinologyDocument20 pagesOverview of Biochemical EndocrinologyGauri KashyapNo ratings yet
- Biocatalysis Questions and AnswersDocument9 pagesBiocatalysis Questions and Answerskumara guruparanNo ratings yet
- Gene transfer using retro and adeno virusesDocument17 pagesGene transfer using retro and adeno virusesVyshali PingleNo ratings yet
- 2020 GKS-G Available Universities & Fields of Study (English)Document348 pages2020 GKS-G Available Universities & Fields of Study (English)Rahma FadhilaNo ratings yet
- NURSING ANAPHY Lec Session #12 - SAS (NEW FORMAT) PDFDocument2 pagesNURSING ANAPHY Lec Session #12 - SAS (NEW FORMAT) PDFKriztel Andrei Lapac NavajaNo ratings yet
- 1. Chuyển hóa carbohydrate (metabolism of carbohydrates) : GlycolysisDocument20 pages1. Chuyển hóa carbohydrate (metabolism of carbohydrates) : GlycolysisAnh Tuyet NguyenNo ratings yet
- Drug DesignDocument38 pagesDrug DesignPhArMaCyGrAdUaTeSNo ratings yet
- Ethical Aspects of BiotechnologyDocument47 pagesEthical Aspects of Biotechnologyalfi alfathana100% (1)
- 473-Article Text-903-1-10-20181112Document122 pages473-Article Text-903-1-10-20181112unaiza100% (1)
- B.N.Bandodkar College Post-Reaccreditation ReportDocument47 pagesB.N.Bandodkar College Post-Reaccreditation Reportsdf6619No ratings yet
- The Chaleging BiofilmDocument58 pagesThe Chaleging BiofilmZayra Coral Ortiz OjedaNo ratings yet
- Genetic Markers: DNA Markers Provide High ResolutionDocument19 pagesGenetic Markers: DNA Markers Provide High ResolutionhotmarulitualinggaNo ratings yet