Professional Documents
Culture Documents
A Review of Anesthetic Effects On Renal Function: Potential Organ Protection
Uploaded by
Sianipar RomulussOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
A Review of Anesthetic Effects On Renal Function: Potential Organ Protection
Uploaded by
Sianipar RomulussCopyright:
Available Formats
In-Depth Topic Review
Nephrology
American Journal of
Am J Nephrol 2017;46:380–389 Published online: November 7, 2017
DOI: 10.1159/000482014
A Review of Anesthetic Effects on Renal
Function: Potential Organ Protection
Negar Motayagheni a Sheshanna Phan b Crystal Eshraghi b Ala Nozari c
Anthony Atala d
a Institute
for Regenerative Medicine (Wake Forest Institute of Regenerative Medicine), Wake Forest School
of Medicine Medical Center Boulevard, Winston-Salem, NC, USA; b Department of Anesthesiology, Division of
Molecular Medicine, UCLA David Geffen School of Medicine, Los Angeles, CA, USA; c Department of Anesthesia,
Critical Care and Pain Medicine, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA;
d Institute of Regenerative Medicine, Wake Forest School of Medicine, Winston-Salem, NC, USA
Keywords tion. Some anesthetic drugs induce anti-inflammatory, anti-
Anesthetic drugs · Ischemia-reperfusion injury · Volatile necrotic, and anti-apoptotic effects. A more thorough
anesthetics · Organ protection · Transplant · Intravenous understanding of anesthetic effects on renal function can
anesthetics · Lipid emulsion · Propofol · Cardioprotection · present a novel approach for developing organ-protective
Renal protection strategies. The aim of this review is to discuss the effects of
different anesthetic drugs on renal function, with particular
focus on IR injury. Many studies have demonstrated the or-
Abstract gan-protective effects of some anesthetic drugs, specifically
Background: Renal protection is a critical concept for anes- propofol, which indicate the potential of some anesthetics
thesiologists, nephrologists, and urologists, since anesthesia to introduce novel organ protective targets. This is not sur-
and renal function are highly interconnected and can poten- prising, since lipid emulsions are major components of pro-
tially interfere with one another. Therefore, a comprehensive pofol, which accumulating data show provide organ protec-
understanding of anesthetic drugs and their effects on renal tive effects against IR injury. Key Messages: Thorough un-
function remains fundamental to the success of renal surger- derstanding of the interaction between anesthetic drugs
ies, especially transplant procedures. Some experimental and renal function remains fundamental to the delivery of
studies have shown that some anesthetics provide protec- safe perioperative care and to optimizing outcomes after re-
tion against renal ischemia/reperfusion (IR) injury, but there nal surgeries, particularly transplant procedures. Anesthet-
is limited clinical evidence. Summary: The effects of anes- ics can be repurposed for organ protection with more infor-
thetic drugs on renal failure are particularly important in the mation about their effects, especially during transplant pro-
context of kidney transplantation, since the conditions of cedures. Here, we review the effects of different anesthetic
preservation following removal profoundly influence the re- drugs – specifically those that contain lipids in their struc-
covery of organ function. Currently, preservation proce- ture, with special reference to IR injury.
dures are typically based on the usage of a cold-storage solu- © 2017 S. Karger AG, Basel
© 2017 S. Karger AG, Basel Negar Motayagheni, MD
Institute for Regenerative Medicine (WFIRM)
391 Technology Way, Wake Forest School of Medicine Medical Center Boulevard
E-Mail karger@karger.com
Winston Salem, NC 27101 (USA)
www.karger.com/ajn E-Mail nmotayag @ Wakehealth.edu, negar.motayagheni @ yahoo.com
Introduction General Anesthesia
Renal ischemia/reperfusion (IR) injury is a leading Volatile Anesthetics
cause of preoperative acute kidney injury (AKI), which Volatile anesthetics are administered to many patients
frequently complicates major vascular, cardiac, trans- subjected to general anesthesia and are an integral part of
plant, and liver surgeries [1]. AKI has been shown to oc- the perioperative period. Methoxyflurane was the first
cur after some major surgeries, raising questions regard- nonflammable halogenated volatile anesthetic gas syn-
ing the role of the operative procedures – including the thesized [3]. Methoxyflurane caused dose-dependent ab-
administration of anesthesia and its effects on renal func- normalities post-surgery, including vasopressin-resistant
tion [1]. polyuria, serum hyperosmolality, hypernatremia, in-
There is contradictory evidence regarding the effects creased concentrations of serum urea nitrogen and inor-
of anesthetics on renal function. Some studies have ganic fluoride, and decreased urinary potassium, sodium,
shown that the administration of some types of anesthe- osmolality, and urea nitrogen concentrations, with clini-
sia during surgery, as well as surgical stress itself, can af- cal toxicity at dosages greater than 90 μmol/L [14]. Con-
fect renal function. Indirect effects are more pronounced sequently, nephrotoxicity induced by methoxyflurane
than the direct effects [2]. However, other studies have was generalized to all halogenated anesthetics. However,
shown that some anesthetic drugs induce anti-inflam- most third-generation inhaled anesthetics are effective
matory, anti-necrotic, and anti-apoptotic effects that and safe [4].
protect against AKI [3]. This raises several questions, in- Fluorinated anesthetics, specifically sevoflurane and
cluding: Can anesthetics attenuate or prevent renal IR enflurane, did not cause deterioration of postoperative
injury? What are the possible mechanisms? How does renal function in patients with preexisting renal issues
the anesthetic technique influence patient outcomes af- [15]; none of the patients needed dialysis or had perma-
ter renal transplantation? Can we repurpose them as or- nent deterioration of renal insufficiency. Furthermore,
gan-protective agents? both animal and human studies have demonstrated that
Identifying appropriate anesthetic technique for renal neither the duration of systemic fluoride exposure nor the
procedures, especially transplantations, is vital (Table 1). fluoride peak values corresponded to anesthetic nephro-
In particular, novel interventions that protect against IR toxicity [3]. In fact, the metabolism of enflurane to inor-
injury are needed to improve early graft function after ganic fluoride during and after surgery did not cause a
kidney transplantation. Available general and local anes- clinically significant level of renal disease or dysfunction
thetics, including third generation inhaled anesthetics, [16].
propofol, and amide-class local anesthetics, are effective Accumulating data show protective effects of some
and safe with a low incidence of side effects. Anesthetics volatile anesthetic drugs against IR injury [17, 18]. In a
seem to exhibit an organ-protective potential via multiple study analyzing data from the past 15 years, pre-injury
different mechanisms, including reducing IR injury administration of a volatile anesthetic was shown to de-
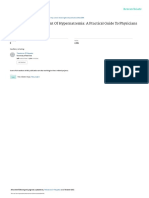
(Fig. 1) [4]. Some researchers propose that these anesthet- crease the impact of IR injury on the heart, brain, and
ics prevent the uncontrolled opening of the mitochon- kidney [19]. Other data demonstrated that volatile agents
drial permeability transition pore after ischemia, which administered shortly after injury can decrease IR injury
leads to the release of pro-apoptotic factors and necrotic [20].
cell death [5]. In particular, propofol, a widely used anes- Additionally, Darwin’s theory of evolution led to the
thetic, has shown potential as a novel organ-protective concept of preconditioning, a mechanism in which brief
agent through its efficient membrane-targeted and cyto- sublethal periods of ischemia provide protection from a
protective effects [4]. This is not surprising since lipid subsequent lethal episode of ischemia and mitigate the
emulsions are major components of propofol, which ac- effect of IR [21]. Subsequently, the organism or the tissue
cumulating data show provide organ protective effects will acquire an “injury-resistant” phenotype for a certain
against IR injury in many organs, such as the heart, kid- period [22]. Interestingly, brief periods of ischemia at the
ney, liver, and intestines [6–13]. onset of reperfusion are associated with cardioprotection,
In this review, we summarize the research history on leading to a decrease in myocardial infarction size [23].
the effects of anesthetic drugs on kidney function, includ- Similarly, isoflurane has been shown to improve remod-
ing cellular mechanisms of anesthetic-mediated protec- eling after coronary artery occlusion in rats [24]. Precon-
tion in different organs (Table 2). ditioning is now a well-established property of volatile
Anesthetic Effects on Renal Function Am J Nephrol 2017;46:380–389 381
DOI: 10.1159/000482014
Table 1. Overview of common anesthetic agents with their primary physiological and adverse effects
Drug Action Adverse effects
Volatile anesthetics
Methoxyflurane Analgesic Respiratory and cardiovascular system depression, renal damage no longer
available for use in the United States
Sevoflurane Anesthetic Raises intracranial pressure
Enflurane Anesthetic Increased risk of seizure activity, malignant hyperthermia
Isoflurane Anesthetic Moderate to severe airway irritability if used as an induction agent
Intravenous anesthetics
Ketamine Anesthetic Analgesic Psychomimetic effects post-surgery
Dexmedetomidine Anesthetic Analgesic Use with caution in patients with preexisting cardiac conduction defects,
bradycardia, hypovolemia
Propofol Anesthetic Respiratory and cardiovascular system depression
Regional anesthetics
Bupivacaine Analgesic Cardiotoxicity
Lidocaine Analgesic
anesthetics, specifically of sevoflurane; these anesthetics Furthermore, sevoflurane can impair kidney function;
are recommended as the agents of choice by the American the inorganic fluoride ions resulting from its defluorina-
Heart Association for high-risk patients. Preconditioning tion and the production of compound A from the reac-
and postconditioning with sevoflurane exert a significant tion with carbon dioxide absorbent have been associated
protective effect against IR injury in the rat lung trans- with nephrotoxicity [28, 29]. Fluoride levels following the
plantation model [25]. administration of isoflurane or halothane increase by 3–5
Other volatile anesthetics exhibit promising post-con- and 1–2 µmol/L, respectively, causing the risk of nephro-
ditioning properties after cardiac surgery. At the basic toxicity to be relatively improbable. Comparatively, des-
level, the myocardial protective effects of sevoflurane in- flurane’s resistance to biodegradation allows even pro-
volve apoptotic mRNA inhibition, neuromodulation, cy- longed exposure to be associated with normal renal func-
tokine/inflammation modulation, redox-sensitive path- tion [28]. In a recent study, researchers stored porcine
ways, endothelial preservation, ion channels, and notch kidneys in the preservation solution Celsior, which was
signaling pathways [26]. These findings open a new field saturated with argon or xenon. Argon-Celsior showed
of investigation for potential therapies aimed to diminish promise in renal transplant preservation by improving
secondary organ injury, as well as transplants. However, early functional recovery, graft quality, and survival, in
more studies are required to assess the magnitude of col- comparison to Xenon-Celsior [30].
lateral protection of other organs. Recent studies have shown that volatile anesthetics
Additionally, researchers have looked at the possibility provide protective effects during and after ischemic and
of adding volatile anesthetics to preservation solutions inflammatory conditions that occur in the perioperative
for renal transplantations. Such anesthetic management period by modulating IR injury and inflammation [3, 15,
aims to maintain the optimum perfusion pressure of the 16, 31]. Researchers found that isoflurane provides pre-
renal allograft in order to preserve its function. Both sevo- conditioning renoprotective effects through anti-inflam-
flurane and enflurane have been shown to undergo bio- matory and anti-apoptotic actions in a rat model [32].
degradation into inorganic fluoride. Evidence of renal Isoflurane may be protecting against renal tubular necro-
concentrating ability and renal tubular injury with tran- sis and inflammation by inducing renal tubular CD73
sient impairment was found in patients who received and adenosine generation, which is dependent on trans-
sevoflurane and enflurane [27], as a serum fluoride con- forming growth factor-beta 1 [33]. Similarly, sevoflurane
centration of 50 µmol/L is the peak nephrotoxicity value. pretreatment enhanced hypoxia-inducible factor-2α ex-
382 Am J Nephrol 2017;46:380–389 Motayagheni/Phan/Eshraghi/Nozari/
DOI: 10.1159/000482014 Atala
Color version available online
Anesthetics
• Volatile
• Regional
• Intravenous
Mitochondria
NF-κB Antiapoptotoc
pathway
iNOS Mitochondria
mPTP opening
Ca2+ Anti
SOD Altered inflammatory
myocardial Ca2+ pathway
flux
ROS Openning of
mitoKATP channel GOX FOX
ATP Apoptotic Neuro GLUT 4
Mitochondria mRNA modulation
Lipid raft
Inflammation Endothelial
modulation preservation
Notch signalling
Attenuated Recovery
Preconditioning Cytoprotective
oxidative from Ca2+
to IRI effect
stress overload
Organ-protective effect
of anesthetics during IRI
Fig. 1. Hypothesized mechanisms for pro-
tective effects of anesthetics.
pression in a mouse model of renal IR injury [34]. An- that sevoflurane preconditioning significantly lessened
other study showed that xenon protects against AKI by the postoperative rise of transaminase levels in patients
activating miR-21 target signaling pathways [35]. undergoing liver resection [37]. Furthermore, a short pe-
Additionally, a recent study simulating liver trans- riod of sevoflurane preconditioning in patients undergo-
plantation in rats investigated the mechanisms by which ing coronary artery bypass graft surgery was shown to
volatile anesthetics yield their organ protective effects, significantly decrease both the release of a myocardial
comparing the protective and antioxidant properties of contractile dysfunction marker and the levels of plasma
sevoflurane and isoflurane [36]. It was found that sevo- cystatin C concentrations [38]. These findings suggest
flurane had significantly better protective and antioxi- that sevoflurane causes improvements in renal and car-
dant effects during both cold preservation and the early diac function following major surgery. Additionally, a re-
phases of organ reperfusion in comparison to isoflurane, cent study suggested that during the early postoperative
suggesting the differential protective effects of common period following kidney transplantation, the estimated
volatile anesthetics [36]. glomerular filtration rate improves significantly when
There is significant clinical evidence for volatile anes- living donors are anesthetized with a volatile anesthetic,
thetic-mediated organ protection. A recent study showed as compared to with propofol [39]. The protective effects
Anesthetic Effects on Renal Function Am J Nephrol 2017;46:380–389 383
DOI: 10.1159/000482014
Table 2. Proposed interactions and mechanisms of anesthetics
Drug Proposed interactions
Volatile anesthetics
Methoxyflurane Nephrotoxicity [14]
Sevoflurane No deterioration of renal function [15]
Protective effect against IR injury [24]
Nephrotoxicity due to production of inorganic fluoride ions and Compound A
Preconditioning renoprotective effects [30]
Enflurane No deterioration of renal function [15]
Isoflurane Preconditioning renoprotective effects [28]
Protected against renal tubular necrosis and inflammation by inducing renal tubular CD73 and adenosine
generation [29]
Intravenous anesthetics
Ketamine Ameliorated the up-regulation of inflammatory pathways and reduction of metabolism caused by
hypoxia [33]
Dexmedetomidine Inhibited oxidative stress and inflammation [53]
Propofol Reduced renal IR injury in rat model [40]
Significantly reduced the incidence and severity of AKI in comparison to sevoflurane [48] and
midazolam [49]
Pretreatment prevented decrease in renal function and an increase in tubular apoptosis by inhibiting
oxidative stress [42]
Pretreatment protected cells against apoptosis induced by IR [46]
Modulated systemic inflammation from IR by decreasing expression of nuclear factor-κB [43]
Mitigated renal IR injury via heme oxygenase-1 expression induction [44]
Attenuated post-AOLT AKI via inhibition of Cx32 function [63]
Promising renoprotective agent in renal transplantation [54]
Regional anesthetics
Bupivacaine Lower toxicity for the recipient and renal allografts during renal transplantation [57]
Lidocaine Lower toxicity for the recipient and renal allografts during renal transplantation [57]
Protection against IR injury via miRNA dysregulation prevention [60]
of volatile anesthetics were also evaluated in a recent One study determined that volatile anesthetics, such as
study that investigated the mechanisms of IR injury, and isoflurane, provide IR injury protection by attenuating
identified one such mechanism as intracellular calcium both inflammation and necrosis [41]. Another study
overload. It was suggested that such anesthetics may pro- showed that preconditioning with xenon had a protec-
tect the myocardium from IR injury by altering myocar- tive effect by preventing renal IR injury from progress-
dial calcium fluxes, preserving myocardial energetics, ing to AKI due to its natural induction of hypoxia-in-
and protecting the region from reactive oxygen species ducible factor, thus yielding potentially important clini-
injury [40]. The relatively higher efficiency of enflurane cal applications [42]. Furthermore, another study
and halothane in comparison to isoflurane regarding investigating histological tubular cell damage in a rabbit
these effects has been explained by their effect on myo- model found that desflurane preconditioning reduced
cardial cells: halothane and enflurane primarily decrease renal IR injury via its protective effect on the kidneys
intracellular calcium levels by directly acting on the sar- [43].
coplasmic reticulum, while isoflurane only decreases Although some volatile anesthetics attenuate AKI,
transsarcolemnal calcium entry (Fig. 1) [40]. multiple studies have shown that propofol, an intrave-
Recently, various studies have shown the protective nous anesthetic with anti-inflammatory properties, may
effects of volatile anesthetics in terms of renal IR injury. attenuate AKI more effectively.
384 Am J Nephrol 2017;46:380–389 Motayagheni/Phan/Eshraghi/Nozari/
DOI: 10.1159/000482014 Atala
Intravenous Anesthetics of inducible nitric oxide synthase, and phosphorylation
Renal IR injury is a risk factor for acute renal failure of inhibitor of κB and nuclear factor-κB [58].
and delayed graft function. Pathogenic factors for renal Propofol may protect cells against apoptosis induced
IR include, but are not limited to, the following: oxidative by IR through a preconditioning effect. In one study, pro-
stress, inflammation, cellular necrosis, and apoptosis pofol attenuated IR injury in LLC-PK1 cells when pre-
[44]. Some anesthetic drugs induce anti-inflammatory, sented either 1 or 24 h before IR or during the recovery
anti-necrotic, and anti-apoptotic effects through differ- period but not when it was added only during ischemia.
ent mechanisms [3]. In a recent study, researchers intra- This effect may have been partially mediated by KATP
venously administered ketamine into fetal sheep before channels [59]. In addition, propofol pretreatment may
inducing hypoxia. Ketamine ameliorated the upregula- modulate bone morphogenetic protein 2 expression to
tion of inflammatory pathways and reduction of metabo- reduce renal oxidative injury and facilitate repair follow-
lism caused by hypoxia [45]. Furthermore, thiopental ing IR injury [60].
pretreatment reduced renal IR injury induced by free rad- In a comparison between patients receiving propofol
icals [46, 47]. Reinforcing this finding, dexmedetomidine or sevoflurane during cardiopulmonary bypass, propofol
has been shown to decrease brain IR injury and inhibit significantly reduced the incidence and severity of AKI
nuclear factor-κB and intercellular adhesion molecule 1 (which was defined using the AKI network criteria as an
expression through the inhibition of oxidative stress and absolute increase in serum creatinine of 0.3 mg/dL, an
inflammation, which are pathogenic factors of IR injury increase ≥150% from the baseline value, or as a urine out-
[48] put of <0.5 mL/kg/h for >6 h within 48 h postoperative)
However, the majority of research studies have fo- in comparison to sevoflurane [61]. This suggests that
cused on the non-anesthetic effects of propofol, such as propofol may be a better renoprotective agent for cardio-
its antioxidant, immunomodulatory, analgesic, and neu- pulmonary bypass than sevoflurane. No relevant clinical
roprotective effects [47]. Propofol has been shown to complications occurred with either anesthetic method,
have protective effects against IR injury in multiple or- and other postoperative outcomes (such as intensive care
gans, including the heart, kidneys, liver, and intestines unit [ICU] stay and in-hospital mortality) were similar
[6–13, 49–52]. between the 2 groups. AKI that occurred in patients who
The antioxidant abilities of propofol significantly re- received propofol was confined to the lowest stage of the
duced IR injury in a rat model of renal IR [53]. Research- AKI Network criteria, while AKI that occurred in pa-
ers have shown that pretreatment with propofol attenu- tients who received sevoflurane varied across the disease
ated the characteristic decrease in renal function and an spectrum. In fact, significantly more patients receiving
increase in tubular apoptosis in male rat kidneys subject- sevoflurane displayed severe renal dysfunction and re-
ed to IR. Propofol protected cells from apoptosis by in- quired dialysis. Measured perioperative changes in se-
hibiting oxidative stress and the corresponding down- rum biomarkers of renal injury and inflammatory me-
stream pathways, such as mitochondrial stress [54]. Pro- diators, and the occurrence of postoperative complica-
pofol may counteract oxidative stress by lowering the tions contributed to the clinical evaluation of patient
formation of F(2)-isoprostane, a marker of oxidative outcomes. C-reactive protein, creatine kinase-MB, and
stress, during transplantation and post-surgery [55]. Pro- neutrophil counts were measured pre- and postopera-
pofol also modulates systemic inflammation resulting tively, and interleukin-1, interleukin-6, and tumor ne-
from IR by decreasing the expression of nuclear factor- crosis factor-α levels were assessed to evaluate the degree
κB, a nuclear transcription factor that plays a key role in of inflammation and AKI [61]. In another study, patients
oxidative stress (Fig. 1) [56]. Propofol may also induce treated with propofol had better ICU survival than those
heme oxygenase-1 expression in order to mitigate renal treated with benzodiazepine. The outcomes for patients
IR injury [57]. Furthermore, propofol can also induce in the ICU that were exclusively treated with propofol or
protection against renal IR injury aggravated by hyper- midazolam were compared for the first 48 h. Patients
glycemia through its antioxidant abilities [58]. In com- treated with propofol had a lower risk of AKI, fluid-re-
parison to etomidate, propofol significantly attenuated lated complications, and less need for RRT compared
tubular damage after reperfusion in hyperglycemic rats. with midazolam [62].
It also preserved superoxide dismutase levels and attenu- It has been suggested that reperfusion-induced en-
ated post-reperfusion increases in levels of myeloperoxi- hancement of the connexin32 (Cx32) gap junction plays
dase, interleukin-1β, tumor necrosis factor-α, production an important role in mediating AKI post-liver transplan-
Anesthetic Effects on Renal Function Am J Nephrol 2017;46:380–389 385
DOI: 10.1159/000482014
tation. In one study, male rats underwent autologous liv- agents that produced the least renal oxidative stress in this
er transplantation (AOLT) with or without selective Cx32 model of common bile duct ligation-induced obstructive
inhibitor, 20-amino-ethoxydiphenyl, or propofol. AOLT jaundice [69]. Free radical injury in renal tissue during
significantly increased renal Cx32 protein expression, transplantation surgery was suggested to have significant
gap junction formation, and oxidative stress, and it im- importance in preventing related AKI.
paired renal function. Propofol inhibited Cx32 function Various clinical studies have helped elucidate the rela-
while attenuating post-AOLT AKI [63]. Another study tionship between anesthesia and clinical AKI. A recent
suggested that propofol exerts a renoprotective effect meta-analysis was conducted for remote ischemic pre-
against AKI after orthotopic liver autotransplantation conditioning (RIPC) trials in patients prior to undergo-
through the upregulation of the nuclear factor, erythroid ing cardiac surgery. Although researchers found that
2-related factor 2, a regulator of the cellular-defense re- there was a significant reduction in AKI when propofol
sponse protection against oxidative injury [64]. A recent was not used, no effects were observed in those who re-
study found that propofol protects against hepatic IR in- ceived both propofol and RIPC during surgery. This sug-
jury through the regulation of mitogen-activated protein gests that propofol may interact with the protective com-
kinase 6 expression by miR-133a-5p [65]. There is exten- ponents of RIPC, thereby improving clinical outcomes
sive supporting data regarding the protective effects of [70]. Furthermore, another recent study compared the
lipid emulsions against IR injury [6–13]. Considering the clinical outcomes of propofol and sevoflurane anesthesia
presence of lipids in propofol, this could provide new in- on postoperative AKI [71]. A multivariable analysis of
sights into organ-protective targets. 4,320 colorectal surgery patients’ medical records re-
Additionally, researchers have evaluated the effec- vealed that the incidence of AKI was significantly higher
tiveness of propofol in preservation solution in a por- in patients who received sevoflurane than in those who
cine autotransplantation model after 45 min of isch- received propofol, suggesting that anesthesia with propo-
emia. Their results demonstrated that propofol is a fol may be associated with improved clinical AKI out-
promising renoprotective agent that may attenuate hy- comes for patients [71]. Additionally, another study con-
pothermic and ischemic AKI in renal transplantation sisting of a randomized placebo-controlled trial investi-
through its antioxidant effects [66]. This mechanism of gated whether dexmedetomidine prevents AKI following
transplantation preservation is driven by propofol’s ac- valvular heart surgery [72]. AKI incidence, morbidity,
tion as a cytoprotective agent and membrane-targeted and ICU stay were all found to be significantly lower for
antioxidant. As such, propofol protects tubular cells patients who received dexmedetomidine than for those
from being affected by hypothermic injury in vitro, and who received the placebo, suggesting that perioperative
the addition of its cyclodextrin complex to the preserva- administration of dexmedetomidine may reduce the in-
tion fluid during machine perfusion delivers it to the cidence and severity of AKI, thus improving clinical out-
kidneys and prevents lipid peroxidation, while dimin- comes for patients undergoing cardiac surgery [72].
ishing the early reperfusion period’s renovascular resis-
tance after transplantation [66]. However, recent clini-
cal trials have suggested that volatile anesthetics, such as Regional Anesthesia
desflurane, used for anesthetic maintenance and preser-
vation during transplantation are associated with better It has been found that the addition of bupivacaine to a
outcomes than intravenous anesthetics [67]. In fact, depolarizing cardioplegia solution reduced cell damage
most currently available volatile anesthetics have been and improved cardiac function after prolonged storage
shown to efficiently preserve hepatic function and blood [73]. Bupivacaine’s ability to decreased cell damage sug-
flow [68]. gests that it may have some protective effects against renal
Furthermore, a recent study involving rats with ob- IR injury.
structive jaundice evaluated the protective effects of vari- In fact, several studies have found that epidural anal-
ous common intravenous anesthetics on renal tissues, gesia (EDA) reduces the incidence of acute renal failure.
and found that the incidence of postoperative AKI was In a study that investigated the effect of EDA on renal
higher in rats with obstructive jaundice than in those blood flow in 13 healthy volunteers, researchers adminis-
without it, and that obstructive jaundice causes renal tis- tered lidocaine 2% without epinephrine to establish a bi-
sue to become sensitive to anesthetic damage [69]. It was lateral T6 epidural sensory block. They found that EDA
found that propofol and ketamine were the 2 anesthetic did not significantly alter renal blood flow [74]. In fact,
386 Am J Nephrol 2017;46:380–389 Motayagheni/Phan/Eshraghi/Nozari/
DOI: 10.1159/000482014 Atala
EDA using lidocaine or bupivacaine is the preferred an- Conclusion
esthesia method in renal transplantations because it dis-
plays lower toxicity for the recipient and renal allograft Certain anesthetics seem to exhibit protective effects
[75]. in patients through anti- inflammatory, anti-necrotic,
Furthermore, a review of trials with randomization of and anti-apoptotic mechanisms. Additionally, intrave-
intraoperative neuraxial blockade showed that EDA re- nous anesthetics, such as propofol, are promising candi-
duces postoperative mortality and other complications, dates for preventing or treating AKI, as well as IR injury.
such as renal failure [76]. This finding is further rein- The effects of anesthetic drugs on renal function are par-
forced by a meta-analysis, which found that EDA, in ad- ticularly important in the context of kidney transplanta-
dition to general anesthesia, reduced the incidence of tion and preservation strategies. Although anesthetics
perioperative acute renal failure in cardiac surgery [77]. may have important clinical implications, our under-
Lidocaine may provide protection against IR injury by standing of the underlying mechanism of their renal pro-
preventing miRNA dysregulation. A recent study found tection is not fully understood. Further research is neces-
that lung IR injury caused miRNA dysregulation, while sary to elucidate the molecular mechanisms of anesthet-
lidocaine reduced these changes [78]. Further studies ics, specifically those that contain lipids in their structure,
should be conducted to examine how lidocaine regulates to enable repurposing them for novel applications, such
miRNA expression. as transplant and organ-protective targets.
In contrast, a recent study found that EDA may be a
risk factor for postoperative AKI after major hepatectomy
[79]. While attempting to elucidate the relationship be- Financial Disclosures
tween anesthesia and clinical AKI, it was found that for
major hepatectomies, AKI incidence was significantly The authors have nothing to disclose.
higher among patients who received EDA as compared to
those who did not, suggesting that EDA may contribute
to negative postoperative clinical AKI outcomes for pa- Disclosure Statement
tients [79]. However, since no significant difference in The authors have no conflicts of interest to declare.
AKI incidence was revealed in patients undergoing minor
hepatectomies, contradictory data suggest that EDA may
have a beneficial impact during some types of surgery, but
Data Source
not others. Further studies are required to elucidate the
clinical outcomes of EDA on renal function in different Research in both human and animals has contributed the data
types of surgery. needed for this study.
References
1 Gullick HD, Raizs LG: Changes in renal con- role in anaesthesia-triggered cellular protec- tralipid-induced cardioprotection against
centrating ability associated with major surgi- tion during ischaemia-reperfusion injury. ischemia-reperfusion injury. Cardiology
cal procedures. N Engl J Med 1960;262:1309– Anaesth Intensive Care 2012;40:46–70. 2016;134:241.
1314. 6 L, Ruffenach G, Kararigas G, et al: Intralipid 10 Motayagheni N, Eghbali M: Reversal of xyla-
2 Burchardi H, Kaczmarczyk G: The effect of protects the heart in late pregnancy against zine-induced bradycardia with intralipid.
anaesthesia on renal function. Eur J Anaes- ischemia/reperfusion injury via Caveolin2/ Cardiology 2016;134:431.
thesiol 1994;11:163–168. STAT3/GSK-3β pathway. J Mol Cell Cardiol 11 Motayagheni N: From bupivacaine to intra-
3 Fukazawa K, Lee HT: Volatile anesthetics and 2017;102:108–116. lipid: Leading edge. J Anesth Clin Res 2016;4:
AKI: risks, mechanisms, and a potential ther- 7 Li J, Motayagheni N, Barakati N, Eghbali M: 00164.
apeutic window. J Am Soc Nephrol 2014; 25: Intralipid protects the heart in late pregnancy 12 Li J, Motayagheni N, Barakati N, Eghbali M:
884–892. against ischemia/reperfusion injury by reduc- Intralipid protects the heart against ischemia/
4 Sellbrant I, Brattwall M, Jildenstål P, War- ing cardiomyocyte apoptosis via Mir122 in- reperfusion injury by reducing cardiomyo-
ren-Stomberg M, Forsberg S, Jakobsson JG: duction. Circ Res 2017;119:A442. cyte apoptosis via miR122 induction in late
Anaesthetics and analgesics; neurocognitive 8 Motayagheni N, Eghbali M: Complete rever- pregnancy. Cardiology 2016;134:313.
effects, organ protection and cancer reoc- sal of xylazine-induced bradycardia with in- 13 Motayagheni N, Sharma S, Li J, Eghbali M:
currence an update. Int J Surg 2016; 34: 41– tralipid in female mice. Circ Res 2016; Implication of miR-1 and miR- 144 in intra-
46. 119:A253 lipid-induced cardioprotection against isch-
5 Andrews DT, Royse C, Royse AG: The mito- 9 Motayagheni N, Phan S, Eshraghi C, Eghbali emia/reperfusion injury. Cardiology 2016;
chondrial permeability transition pore and its M: Inhibition of leptin receptor abolishes in- 134:430.
Anesthetic Effects on Renal Function Am J Nephrol 2017;46:380–389 387
DOI: 10.1159/000482014
14 Cousins MJ, Mazze RI: Methoxyflurane 27 Gentz BA, Malan TP: Renal toxicity with 41 Lee HT, Ota-Setlik A, Fu Y, Nasr SH, Emala
nephrotoxicity. A study of dose response in sevoflurane: a storm in a teacup? Drugs 2001; CW: Differential protective effects of volatile
man. JAMA 1973;225:1611–1616. 61;2155–2162. anesthetics against renal ischemia-reperfu-
15 Conzen PF, Nuscheler M, Melotte A, et al: Re- 28 Baxi V, Jain A, Dasgupta D: Anaesthesia for sion injury in vivo. Anesthesiology 2004;101:
nal function and serum fluoride concentra- renal transplantation: an update. Indian J An- 1313–1324.
tions in patients with stable renal insufficien- aesth 2009;53:139–147. 42 Daqing M, Lim T, Xu J, et al: Xenon precon-
cy after anesthesia with sevoflurane or enflu- 29 Kobayashi Y, Ochiai R, Takeda J, Sekiguchi H, ditioning protects against renal ischemic-re-
rane. Anesth Analg 1995;81:569–575. Fukushima K: Serum and urinary inorganic perfusion injury via hif-1alpha activation. J
16 Cousins MJ, Greenstein LR, Hitt BA, Mazze fluoride concentrations after prolonged inha- Am Soc Nephrol 2009;20:713–720.
RI: Metabolism and renal effects of enflurane lation of sevoflurane in humans. Anesth 43 Guye ML, Mc Gregor B, Weil G, Arnal F, Piri-
in man. Anesthesiology 1976;44:44–53. Analg 1992;74:753–757. ou V: [Ischaemic and pharmacologic precon-
17 Hashiguchi H, Morooka H, Miyoshi H, et al: 30 Faure A, Bruzzese L, Steinberg JG, et al: Ef- ditioning: desflurane reduces renal reperfu-
Isoflurane protects renal function against fectiveness of pure argon for renal transplant sion injury in rabbits]. Ann Fr Anesth Rean-
ischemia and reperfusion through inhibition preservation in a preclinical pig model of het- im. 2010;29:518–523.
of protein kinases, JNK and ERK. Anesth erotopic autotransplantation. J Transl Med 44 Basile DP, Anderson MD, Sutton TA: Patho-
Analg 2005;101:1584–1589. 2016;14:40. physiology of acute kidney injury. Compr
18 Vianna PT, Castiglia YM, Braz JR, et al: Remi- 31 Cai J, Xu R, Yu X, Fang Y, Ding X: Volatile Physiol 2012;2:1303–1353.
fentanil, isoflurane, and preconditioning at- anesthetics in preventing acute kidney injury 45 Chang EI, Zarate MA, Rabaglino MB, et al:
tenuate renal ischemia/reperfusion injury in after cardiac surgery: a systematic review and Ketamine suppresses hypoxia-induced in-
rats. Transplant Proc 2009;41:4080–4082. meta-analysis. J Thorac Cardiovasc Surg flammatory responses in the late-gestation
19 Bracco D: Post-conditioning: promising an- 2014;148:3127–3136. ovine fetal kidney cortex. J Physiol 2016; 594:
swers and more questions. Crit Care 2012;16: 32 Liang Y, Li Z, Mo N, et al: Isoflurane precon- 1295–1310.
180. ditioning ameliorates renal ischemia-reperfu- 46 Yuzer H, Yuzbasioglu MF, Ciralik H, et al: Ef-
20 Steurer MP, Steurer MA, Baulig W, et al: Late sion injury through antiinflammatory and fects of intravenous anesthetics on renal isch-
pharmacologic conditioning with volatile an- antiapoptotic actions in rats. Biol Pharm Bull emia/reperfusion injury. Ren Fail 2009; 31:
esthetics after cardiac surgery. Crit Care 2012; 2014;37:1599–1605. 290–296.
16:R191. 33 Kim M, Ham A, Kim JY, et al: The volatile 47 Dogan Z, Yuzbasioglu MF, Kurutas EB, et al:
21 Murry CE, Jennings RB, Reimer KA: Precon- anesthetic isoflurane induces ecto-5’-nucleo- Thiopental improves renal ischemia-reperfu-
ditioning with ischemia: a delay of lethal cell tidase (CD73) to protect against renal isch- sion injury. Ren Fail 2010;32:391–395.
injury in ischemic myocardium. Circulation emia and reperfusion injury. Kidney Int 2013; 48 Li Y, Liu S: The effect of dexmedetomidine on
1986;74:1124–1136. 84:90–103. oxidative stress response following cerebral
22 Gidday JM: Cerebral preconditioning and 34 Zheng B, Zhan Q, Chen J, Xu H, He Z: Sevo- ischemia-reperfusion in rats and the expres-
ischaemic tolerance. Nat Rev Neurosci 2006; flurane pretreatment enhance HIF-2α expres- sion of intracellular adhesion molecule-1
7:437–448. sion in mice after renal ischemia/reperfusion (ICAM-1) and S100B. Med Sci Monit 2017;
23 Zhao ZQ, Corvera JS, Halkos ME, et al: Inhi- injury. Int J Clin Exp Pathol 2015; 8: 13114– 23:867–873.
bition of myocardial injury by ischemic post- 13119. 49 Vasileiou I, Xanthos T, Koudouna E, et al:
conditioning during reperfusion: comparison 35 Jia P, Teng J, Zou J, et al: Xenon protects Propofol: a review of its non-anaesthetic ef-
with ischemic preconditioning. Am J Physiol against septic acute kidney injury via miR-21 fects. Eur J Pharmacol 2009;605:1–8.
Heart Circ Physiol 2003;285:H579–H588. target signaling pathway. Crit Care Med 2015; 50 Eroglu A: The effect of intravenous anesthet-
24 Feng J, Fischer G, Lucchinetti E, et al: Infarct- 43:e250–e259. ics on ischemia-reperfusion injury. Biomed
remodeled myocardium is receptive to pro- 36 Dal Molin SZ, Kruel CR, De fraga RS, Alboim Res Int 2014;2014:821513.
tection by isoflurane postconditioning: role of C, De oliveira JR, Alvares-da-silva MR: Dif- 51 Erturk E: Ischemia-reperfusion injury and
protein kinase B/Akt signaling. Anesthesiol- ferential protective effects of anaesthesia with volatile anesthetics. Biomed Res Int 2014;
ogy 2006;104:1004–1014. sevoflurane or isoflurane: an animal experi- 2014:526301.
25 Ohsumi A, Marseu K, Slinger P, et al: Sevoflu- mental model simulating liver transplanta- 52 Kato R, Foex P: Myocardial protection by an-
rane attenuates ischemia-reperfusion injury tion. Eur J Anaesthesiol 2014;31:695–700. esthetic agents against ischemia-reperfusion
in a rat lung transplantation model. Ann Tho- 37 Beck-Schimmer B, Breitenstein S, Bonvini injury: an update for anesthesiologists. Can J
rac Surg 2017;103:1578–1586. JM, et al: Protection of pharmacological post- Anaesth 2002;49:777–791.
26 Fleisher LA, Beckman JA, Brown KA, et al: conditioning in liver surgery: results of a pro- 53 Yuzbasioglu MF, Aykas A, Kurutas EB, Sa-
ACC/AHA 2007 Guidelines on periopera- spective randomized controlled trial. Ann hinkanat T: Protective effects of propofol
tive cardiovascular evaluation and care for Surg 2012;256:837–844; discussion 844–845. against ischemia/reperfusion injury in rat
noncardiac surgery: executive summary: a 38 Julier K, da Silva R, Garcia C, et al: Precondi- kidneys. Ren Fail 2010;32:578–583.
report of the American College of Cardiol- tioning by sevoflurane decreases biochemical 54 Li Y, Zhong D, Lei L, et al: Propofol prevents
ogy/American Heart Association Task markers for myocardial and renal dysfunction renal ischemia-reperfusion injury via inhibit-
Force on Practice Guidelines (writing com- in coronary artery bypass graft surgery: a dou- ing the oxidative stress pathways. Cell Physiol
mittee to revise the 2002 guidelines on ble-blinded, placebo-controlled, multicenter Biochem 2015;37:14–26.
perioperative cardiovascular evaluation for study. Anesthesiology 2003;98:1315–1327. 55 Basu S, Meisert I, Eggensperger E, Krieger E,
noncardiac surgery) developed in collabo- 39 Lee JH, Joo DJ, Kim JM, Park JH, Kim YS, Koo Krenn CG: Time course and attenuation of
ration with the American Society of Echo- BN: Preconditioning effects of the anesthetic ischaemia-reperfusion induced oxidative in-
cardiography, American Society of Nuclear administered to the donor on grafted kidney jury by propofol in human renal transplanta-
Cardiology, Heart Rhythm Society, Society function in living donor kidney transplanta- tion. Redox Rep 12007;2:195–202.
of Cardiovascular Anesthesiologists, Soci- tion recipients. Minerva Anestesiol 2013; 79: 56 Sanchez-Conde P, Rodriguez-Lopez JM,
ety for Cardiovascular Angiography and 504–514. Nicolas JL, et al: The comparative abilities of
Interventions, Society for Vascular Medi- 40 Louvier N, Lançon JP: [Do halogenated anes- propofol and sevoflurane to modulate inflam-
cine and Biology, and Society for Vascular thetics protect from ischemic and reperfusion mation and oxidative stress in the kidney after
Surgery. J Am Coll Cardiol 2007; 50: 1707– myocardial injuries?]. Ann Fr Anesth Reanim aortic cross-clamping. Anesth Analg 2008;
1732. 1994;13:690–698. 106:371–378.
388 Am J Nephrol 2017;46:380–389 Motayagheni/Phan/Eshraghi/Nozari/
DOI: 10.1159/000482014 Atala
57 Wang HH, Zhou HY, Chen CC, Zhang XL, Nrf2 in rats. Mol Med Rep 2015; 11: 3962– lowing valvular heart surgery. Kidney Int
Cheng G: Propofol attenuation of renal isch- 3968. 2016;89:1164.
emia/reperfusion injury involves heme oxy- 65 Hao W, Zhao ZH, Meng QT, et al: Propofol 73 Ross JD, Ripper R, Law WR, et al: Adding
genase-1. Acta Pharmacol Sin 2007;28:1175– protects against hepatic ischemia/reperfusion bupivacaine to high-potassium cardioplegia
1180. injury via miR-133a-5p regulating the expres- improves function and reduces cellular dam-
58 Yoo YC, Yoo KJ, Lim BJ, et al: Propofol at- sion of MAPK6. Cell Biol Int 2017; 41:495–504. age of rat isolated hearts after prolonged, cold
tenuates renal ischemia-reperfusion injury 66 Snoeijs MG, Vaahtera L, de Vries EE, et al: storage. Anesthesiology 2006;105:746–752.
aggravated by hyperglycemia. J Surg Res 2013; Addition of a water-soluble propofol formu- 74 Suleiman MY, Passannante AN, Onder RL,
183:783–791. lation to preservation solution in experimen- Greene-Helms WF, Perretta SG: Alteration of
59 Assad AR, Delou JM, Fonseca LM, et al: The tal kidney transplantation. Transplantation renal blood flow during epidural anesthesia in
role of KATP channels on propofol precondi- 2011;92:296–302. normal subjects. Anesth Analg 1997;84:1076–
tioning in a cellular model of renal ischemia- 67 Ko JS, Gwak MS, Choi SJ, et al: The effects of 1080.
reperfusion. Anesth Analg 2009; 109: 1486– desflurane and propofol-remifentanil on 75 Solonynko I, Loba M, Orel J, Kobza I, Zhuk R,
1492. postoperative hepatic and renal functions af- Yeliseev G: Renal transplantation – choice of
60 Yang S, Chou WP, Pei L: Effects of propofol ter right hepatectomy in liver donors. Liver anesthesia. Wiad Lek 1997; 50(suppl 1 pt
on renal ischemia/reperfusion injury in rats. Transpl 2008;14:1150–1158. 1):447–448.
Exp Ther Med 2013;6:1177–1183. 68 Kotake Y: Anesthetic protection against he- 76 Rodgers A, Walker N, Schug S, et al: Reduc-
61 Yoo YC, Shim JK, Song Y, Yang SY, Kwak YL: patic injury. Masui 2006;55:570–578. tion of postoperative mortality and morbidity
Anesthetics influence the incidence of acute 69 Hatipoglu S, Yildiz H, Bulbuloglu E, et al: Pro- with epidural or spinal anaesthesia: results
kidney injury following valvular heart sur- tective effects of intravenous anesthetics on from overview of randomised trials. BMJ
gery. Kidney Int 2014;86:414–422. kidney tissue in obstructive jaundice. World J 2000;321:1493.
62 Leite TT, Macedo E, Martins Ida S, Neves Gastroenterol 2014;20:3320–3326. 77 Bignami E, Landoni G, Biondi-Zoccai GG, et
FM, Liborio AB: Renal outcomes in critical- 70 Pierce B, Bole I, Patel V, Brown DL: Clinical al: Epidural analgesia improves outcome in
ly ill patients receiving propofol or midazol- outcomes of remote ischemic precondition- cardiac surgery: a meta-analysis of random-
am. Clin J Am Soc Nephrol 2015; 10: 1937– ing prior to cardiac surgery: a meta-analysis ized controlled trials. J Cardiothorac Vasc
1945. of randomized controlled trials. J Am Heart Anesth 2010;24:586–597.
63 Luo C, Yuan D, Li X, et al: Propofol attenu- Assoc 2017;6:e004666. 78 Rancan L, Simon C, Marchal-Duval E, et al:
ated acute kidney injury after orthotopic liver 71 Bang JY, Lee J, Oh J, Song JG, Hwang GS: The Lidocaine administration controls microR-
transplantation via inhibiting gap junction Influence of propofol and sevoflurane on NAs alterations observed after lung ischemia-
composed of connexin 32. Anesthesiology acute kidney injury after colorectal surgery: a reperfusion injury. Anesth Analg 2016; 123:
2015;122:72–86. retrospective cohort study. Anesth Analg 1437–1447.
64 Ge M, Luo G, Yao W, et al: Propofol pretreat- 2016;123:363–370. 79 Kambakamba P, Slankamenac K, Tschuor C,
ment attenuates remote kidney injury in- 72 Xue F, Zhang W, Chu HC: Assessing periop- et al: Epidural analgesia and perioperative
duced by orthotopic liver autotransplanta- erative dexmedetomidine reduces the inci- kidney function after major liver resection. Br
tion, which is correlated with the activation of dence and severity of acute kidney injury fol- J Surg 2015;102:805–812.
Anesthetic Effects on Renal Function Am J Nephrol 2017;46:380–389 389
DOI: 10.1159/000482014
You might also like
- Endocrine Surgery: Butterworths International Medical Reviews: SurgeryFrom EverandEndocrine Surgery: Butterworths International Medical Reviews: SurgeryI. D. A. JohnstonNo ratings yet
- Neostigmine Administration After Spontaneous Recovery To A Train-of-Four Ratio of 0.9 To 1.0Document11 pagesNeostigmine Administration After Spontaneous Recovery To A Train-of-Four Ratio of 0.9 To 1.0Juan Jose Velasquez GutierrezNo ratings yet
- Preoperative Evaluation Before NoncardiacDocument16 pagesPreoperative Evaluation Before NoncardiacHercilioNo ratings yet
- Preoperative Evaluation Before Noncardiac Surgery.Document16 pagesPreoperative Evaluation Before Noncardiac Surgery.Paloma AcostaNo ratings yet
- PracticeJournal1 PutriMaharaniAndes 19-0010Document8 pagesPracticeJournal1 PutriMaharaniAndes 19-0010putri maharani andesNo ratings yet
- Preope Mayo Clinic 2020Document16 pagesPreope Mayo Clinic 2020claauherreraNo ratings yet
- Effect of Anxiolytic Dose On Incidence of Intraoperative Nausea and Vomiting in CS Under Spinal AnesthesiaDocument8 pagesEffect of Anxiolytic Dose On Incidence of Intraoperative Nausea and Vomiting in CS Under Spinal AnesthesiaCentral Asian StudiesNo ratings yet
- Peripheral Nerve Stimulation For Painful Mononeuropathy Secondary To Leprosy: A 12-Month Follow-Up StudyDocument6 pagesPeripheral Nerve Stimulation For Painful Mononeuropathy Secondary To Leprosy: A 12-Month Follow-Up StudySartika Ayu NingsihNo ratings yet
- International Journal of Anesthetics and Anesthesiology Ijaa 7 118Document7 pagesInternational Journal of Anesthetics and Anesthesiology Ijaa 7 118Ferdy RahadiyanNo ratings yet
- Peripheral Regional Anaesthesia and Outcome: Lessons Learned From The Last 10 YearsDocument14 pagesPeripheral Regional Anaesthesia and Outcome: Lessons Learned From The Last 10 YearsPrisky ChriselawatiNo ratings yet
- 8a. General AnaesthesiaDocument28 pages8a. General Anaesthesiamatchees-gone rogueNo ratings yet
- Ne W Engl and Journal MedicineDocument11 pagesNe W Engl and Journal Medicinea4agarwalNo ratings yet
- Anest GGGDocument10 pagesAnest GGGEdmar José Alves Dos SantosNo ratings yet
- Management of Erectile Dysfunction Post-Radical ProstatectomyDocument15 pagesManagement of Erectile Dysfunction Post-Radical ProstatectomyKeka QuijanoNo ratings yet
- Medicine: Effect of Magnesium Sulfate On Renal Colic PainDocument8 pagesMedicine: Effect of Magnesium Sulfate On Renal Colic PainJunaidy TahirNo ratings yet
- Jurnal Anes 3Document10 pagesJurnal Anes 3Ari SamadNo ratings yet
- 2015 MC Lean Et Al. Dose-Dependent Association Between Intermediate-Acting NMBA and Postop Respi C° AnesthesiologyDocument13 pages2015 MC Lean Et Al. Dose-Dependent Association Between Intermediate-Acting NMBA and Postop Respi C° AnesthesiologyZinar PehlivanNo ratings yet
- Perioperative Antibiotic Use in Sleep Surgery: Clinical RelevanceDocument10 pagesPerioperative Antibiotic Use in Sleep Surgery: Clinical RelevanceBRENDA VANESSA TREVIZO ESTRADANo ratings yet
- Anesthesia Technique and Mortality 2016Document8 pagesAnesthesia Technique and Mortality 2016John Portales ArmasNo ratings yet
- Nonopioid, Multimodal Analgesia As First-Line Therapy After Otolaryngology Operations: Primer On Nonsteroidal Anti-Inflammatory Drugs (Nsaids)Document8 pagesNonopioid, Multimodal Analgesia As First-Line Therapy After Otolaryngology Operations: Primer On Nonsteroidal Anti-Inflammatory Drugs (Nsaids)MUHAMMAD ANWARNo ratings yet
- Nerve-Sparing Radical Hysterectomy in Cervical CancerDocument6 pagesNerve-Sparing Radical Hysterectomy in Cervical CancerdenisdeniNo ratings yet
- Pain Management in Enhanced Recovery After Surgery (ERAS) ProtocolsDocument8 pagesPain Management in Enhanced Recovery After Surgery (ERAS) ProtocolsbokobokobokanNo ratings yet
- Pain Management After CraniotomyDocument17 pagesPain Management After CraniotomyTika HandayaniNo ratings yet
- JPM 13 00586 v2Document14 pagesJPM 13 00586 v2Çağdaş BaytarNo ratings yet
- Upper Extremity Regional Anesthesia Techniques A Comprehensive Review For Clinical Anesthesiologists BestPracResAnest 2020Document17 pagesUpper Extremity Regional Anesthesia Techniques A Comprehensive Review For Clinical Anesthesiologists BestPracResAnest 2020RicardoNo ratings yet
- Randomized Clinical Trial Investigating The Stress Response From Two Different Methods of Analgesia After Laparoscopic Colorectal SurgeryDocument7 pagesRandomized Clinical Trial Investigating The Stress Response From Two Different Methods of Analgesia After Laparoscopic Colorectal Surgeryvalerio.messinaNo ratings yet
- Stress Periop MecDocument6 pagesStress Periop MecAnca OuatuNo ratings yet
- Articulo 2017Document8 pagesArticulo 2017LUCIA BELLOSO GONZÁLEZNo ratings yet
- 03 - Neurotoxicity in OncologyDocument17 pages03 - Neurotoxicity in OncologygorklanNo ratings yet
- Wilson Smith2017Document28 pagesWilson Smith2017FaisalNo ratings yet
- Local Anesthesia .2Document8 pagesLocal Anesthesia .2Ahlan SyahrezaNo ratings yet
- Journal of Clinical AnesthesiaDocument2 pagesJournal of Clinical AnesthesiatsalasaNo ratings yet
- Oral Oncology: Mark K. Wax, James AzziDocument4 pagesOral Oncology: Mark K. Wax, James Azzicr89omfNo ratings yet
- Anaesthesia - 2021 - Desai - Local Anaesthetic Adjuncts For Peripheral Regional Anaesthesia A Narrative ReviewDocument10 pagesAnaesthesia - 2021 - Desai - Local Anaesthetic Adjuncts For Peripheral Regional Anaesthesia A Narrative Revieweralp cevikkalpNo ratings yet
- Veterinary Neurologic Rehabilitation The Rationale For ADocument9 pagesVeterinary Neurologic Rehabilitation The Rationale For ACarolina Vargas VélezNo ratings yet
- Otolaryngol - Head Neck Surg - 2010 - Aynehchi - Systematic Review of Laryngeal Reinnervation TechniquesDocument11 pagesOtolaryngol - Head Neck Surg - 2010 - Aynehchi - Systematic Review of Laryngeal Reinnervation TechniquesAlejandra BenavidezNo ratings yet
- Cerebral Oximetry Monitoring To Maintain Normal Cerebral ExplainDocument11 pagesCerebral Oximetry Monitoring To Maintain Normal Cerebral ExplainDaniel EllerNo ratings yet
- Hydrodissection of Peripheral Nerve EntrapmentsDocument7 pagesHydrodissection of Peripheral Nerve Entrapmentsadam.stenmanNo ratings yet
- Pharma in ElderlyDocument8 pagesPharma in ElderlyJoanna GlezNo ratings yet
- The Effect of IM Ketorolac Tromethamine On Bleeding Time: A Prospective, Interventional, Controlled StudyDocument3 pagesThe Effect of IM Ketorolac Tromethamine On Bleeding Time: A Prospective, Interventional, Controlled StudyDaniel TelloNo ratings yet
- Impact of Intra-Operative Dexamethasone After Scheduled Cesarean Delivery: A Retrospective StudyDocument8 pagesImpact of Intra-Operative Dexamethasone After Scheduled Cesarean Delivery: A Retrospective StudyMiftah Furqon AuliaNo ratings yet
- StrongerDocument19 pagesStrongeryoga.syanNo ratings yet
- Articulo - Anestesia CX UrologicaDocument5 pagesArticulo - Anestesia CX UrologicaNFSOTNo ratings yet
- 4 Regional Anaesthesia Versus GeneralDocument15 pages4 Regional Anaesthesia Versus GeneralMila-Cub SanNo ratings yet
- Introduction to Regional Anesthesia TechniquesDocument6 pagesIntroduction to Regional Anesthesia TechniquesMarcela VelezNo ratings yet
- Drugs OrthodDocument9 pagesDrugs OrthoddrmeenugargNo ratings yet
- Interventional Medications in RenalDocument9 pagesInterventional Medications in RenalAlvaro GaldosNo ratings yet
- Cesarean Section Under Local AnesthesiaDocument2 pagesCesarean Section Under Local AnesthesiaaksinuNo ratings yet
- Jurnal Progesteron THDPDocument7 pagesJurnal Progesteron THDPsalmaNo ratings yet
- Completed Research PaperDocument14 pagesCompleted Research Paperapi-545708059No ratings yet
- Jur DingDocument11 pagesJur DingI'am AjhaNo ratings yet
- Hao, K. 2019. Application of Ozone Therapy in Interventional MedicineDocument4 pagesHao, K. 2019. Application of Ozone Therapy in Interventional MedicineMoi RoksNo ratings yet
- Non Opioid DrugsDocument43 pagesNon Opioid DrugsSlamet KatibNo ratings yet
- Pone 0287833Document19 pagesPone 0287833mazfeik8No ratings yet
- Research ArticleDocument7 pagesResearch ArticleYosia KevinNo ratings yet
- EwanDocument3 pagesEwanEdward Joseph AcostaNo ratings yet
- Compartement SyndromeDocument6 pagesCompartement SyndromeAnna ApsariNo ratings yet
- C. Cozowicz, J. Poeran, N. Zubizarreta, J. Liu, S. M. Weinstein, L. Pichler, M. Mazumdar and S. G. MemtsoudisDocument10 pagesC. Cozowicz, J. Poeran, N. Zubizarreta, J. Liu, S. M. Weinstein, L. Pichler, M. Mazumdar and S. G. MemtsoudisAnonymous wdmpgxNo ratings yet
- The Scalp Block For Postoperative Pain Control in Craniosynostosis Surgery: A Case Control StudyDocument8 pagesThe Scalp Block For Postoperative Pain Control in Craniosynostosis Surgery: A Case Control StudyTheresa JulietNo ratings yet
- Ibuprofen DissertationDocument5 pagesIbuprofen DissertationPsychologyPaperWritingServiceFayetteville100% (1)
- Thrombosisuk Anticoagulation Covid 19Document1 pageThrombosisuk Anticoagulation Covid 19Sianipar RomulussNo ratings yet
- BDR-BDK PELAYANAN MEDIK UPT RS INDRAPURA OKTOBER 2021 Fix3Document1 pageBDR-BDK PELAYANAN MEDIK UPT RS INDRAPURA OKTOBER 2021 Fix3Sianipar RomulussNo ratings yet
- WKSTD 13Document34 pagesWKSTD 13Sianipar RomulussNo ratings yet
- Multimodal Analgesia in Cancer Pain Management (Final 2)Document61 pagesMultimodal Analgesia in Cancer Pain Management (Final 2)Laboratorium RSBMNo ratings yet
- AbcDocument8 pagesAbcMinaz PatelNo ratings yet
- Nutrisi EnteralDocument11 pagesNutrisi EnteralFirmansyah Chaniago100% (2)
- Anaesthesia and Renal Disease: DR Cavin Gray Sheffield Teaching HospitalsDocument33 pagesAnaesthesia and Renal Disease: DR Cavin Gray Sheffield Teaching HospitalsSianipar RomulussNo ratings yet
- AnestesiDocument9 pagesAnestesiSianipar RomulussNo ratings yet
- N2O-O2 inhalation sedation for ranula treatment in anxious pediatric patientDocument9 pagesN2O-O2 inhalation sedation for ranula treatment in anxious pediatric patientnovywardanaNo ratings yet
- AND Renin by The CatDocument7 pagesAND Renin by The CatSianipar RomulussNo ratings yet
- PDFDocument2 pagesPDFSianipar RomulussNo ratings yet
- Patient Positioning During AnaesthesiaDocument6 pagesPatient Positioning During AnaesthesiaDarman ZulfikarNo ratings yet
- Abstract Ica WordDocument3 pagesAbstract Ica WordSianipar RomulussNo ratings yet
- A Review of Anesthetic Effects On Renal Function: Potential Organ ProtectionDocument10 pagesA Review of Anesthetic Effects On Renal Function: Potential Organ ProtectionSianipar RomulussNo ratings yet
- JURNAL UNTUK JURDING Hypernatremia in Critically Ill PtsDocument10 pagesJURNAL UNTUK JURDING Hypernatremia in Critically Ill PtsSufiyaLNo ratings yet
- Arya PDFDocument26 pagesArya PDFRahmawati JuliaNo ratings yet
- Guide to Evaluating and Treating HypernatremiaDocument36 pagesGuide to Evaluating and Treating HypernatremiaSianipar RomulussNo ratings yet
- Comparison of Cholesterol Plasma Concentration and Procalcitonin Level As Predictor Worsening of SepsisDocument14 pagesComparison of Cholesterol Plasma Concentration and Procalcitonin Level As Predictor Worsening of SepsisSianipar RomulussNo ratings yet
- Guidelines For Perioperative Care in Elective ColoDocument37 pagesGuidelines For Perioperative Care in Elective ColoSianipar RomulussNo ratings yet
- 4 Cns Oxycodone Guidelines - June2011Document2 pages4 Cns Oxycodone Guidelines - June2011Sianipar RomulussNo ratings yet
- The Difficult Airway Management in Adult Critical Care: 5 May 2014 J MatsheDocument60 pagesThe Difficult Airway Management in Adult Critical Care: 5 May 2014 J MatsheSianipar RomulussNo ratings yet
- Heart Failure: Definition, Etiology and PathophysiologyDocument108 pagesHeart Failure: Definition, Etiology and PathophysiologySianipar RomulussNo ratings yet
- Cellulitis Skinabscesscareguideline PDFDocument3 pagesCellulitis Skinabscesscareguideline PDFSianipar RomulussNo ratings yet
- Penanganan Pre-Eklampsia-Dr. VioDocument9 pagesPenanganan Pre-Eklampsia-Dr. VioSianipar RomulussNo ratings yet
- Handbook (Chemistry Workshop)Document34 pagesHandbook (Chemistry Workshop)DocsNo ratings yet
- Fat-Containing Liver Lesions On Imaging: Detection and Differential DiagnosisDocument10 pagesFat-Containing Liver Lesions On Imaging: Detection and Differential DiagnosisOana AndreeaNo ratings yet
- Best Practices in Health and NutritionDocument5 pagesBest Practices in Health and NutritionMary Rose Fernando AngelesNo ratings yet
- Beatriz Echeverri ResumeDocument1 pageBeatriz Echeverri Resumeapi-436063355No ratings yet
- GA Refresher January 2017Document60 pagesGA Refresher January 2017ajay kumarNo ratings yet
- 2 51 1588072528 6.ijmpsapr20206Document14 pages2 51 1588072528 6.ijmpsapr20206TJPRC PublicationsNo ratings yet
- Barangay Health Services NC IIDocument113 pagesBarangay Health Services NC IIMairem Barruga100% (2)
- October 23, 2015 Strathmore TimesDocument28 pagesOctober 23, 2015 Strathmore TimesStrathmore TimesNo ratings yet
- CH 6 - Therapeutic Communication-Test-Bank-Tank PDFDocument13 pagesCH 6 - Therapeutic Communication-Test-Bank-Tank PDFtim100% (1)
- Electrical Safety Lab ExperimentDocument16 pagesElectrical Safety Lab ExperimentsheherbanoNo ratings yet
- Bacani vs. NACOCO GR No. L-9657 November 29, 1956Document7 pagesBacani vs. NACOCO GR No. L-9657 November 29, 1956jhn012No ratings yet
- 2013 State of Oklahoma EMS ProtocolsDocument493 pages2013 State of Oklahoma EMS ProtocolsMarian Ioan-Lucian100% (1)
- Bedlam Times Article PhotosDocument18 pagesBedlam Times Article PhotosMarcusFelsmanNo ratings yet
- Ethical - Legal and Social Issues in Medical InformaticsDocument321 pagesEthical - Legal and Social Issues in Medical InformaticsHeriberto Aguirre MenesesNo ratings yet
- Cleaning Standards For WashroomsDocument24 pagesCleaning Standards For WashroomsAalok PandeyNo ratings yet
- Epidemiological Characterization of Tibial Plateau FracturesDocument7 pagesEpidemiological Characterization of Tibial Plateau FracturesArmando RodriguezNo ratings yet
- Drug Interactions HandoutDocument5 pagesDrug Interactions HandoutBhavin DesaiNo ratings yet
- NCMH Lisdexamphetamine LeafletDocument2 pagesNCMH Lisdexamphetamine LeafletAkram IshtiwiNo ratings yet
- Essay 3 Acid Rain KraynyayaDocument9 pagesEssay 3 Acid Rain Kraynyayaapi-512016052No ratings yet
- Key Skills For Hospital DoctorsDocument3 pagesKey Skills For Hospital DoctorsMustafaNo ratings yet
- DiphtheriaDocument18 pagesDiphtheriaShishir ShresthaNo ratings yet
- Prof. Dr. Hamid Jan - Vitamin D and Immunity-230415Document39 pagesProf. Dr. Hamid Jan - Vitamin D and Immunity-230415Santi SuratinNo ratings yet
- Presentation 3 - DR Curran - 0Document41 pagesPresentation 3 - DR Curran - 0শরীফ উল কবীরNo ratings yet
- Course Reflection PaperDocument8 pagesCourse Reflection Paperapi-299783298No ratings yet
- PSORIASISDocument9 pagesPSORIASISDianne BernardoNo ratings yet
- Public Health Nutrition Career OptionsDocument2 pagesPublic Health Nutrition Career OptionsZaka RehmanNo ratings yet
- Electroplating IndustryDocument5 pagesElectroplating IndustryRangasamyNo ratings yet
- Amery Hill School NewsletterDocument24 pagesAmery Hill School NewsletterboredokNo ratings yet
- OPD JournalDocument2 pagesOPD JournalRalph Elvin MacanlalayNo ratings yet
- Business Communication: Dabur India LTDDocument30 pagesBusiness Communication: Dabur India LTDshashank nutiNo ratings yet
- LIT: Life Ignition Tools: Use Nature's Playbook to Energize Your Brain, Spark Ideas, and Ignite ActionFrom EverandLIT: Life Ignition Tools: Use Nature's Playbook to Energize Your Brain, Spark Ideas, and Ignite ActionRating: 4 out of 5 stars4/5 (402)
- The Age of Magical Overthinking: Notes on Modern IrrationalityFrom EverandThe Age of Magical Overthinking: Notes on Modern IrrationalityRating: 4 out of 5 stars4/5 (17)
- By the Time You Read This: The Space between Cheslie's Smile and Mental Illness—Her Story in Her Own WordsFrom EverandBy the Time You Read This: The Space between Cheslie's Smile and Mental Illness—Her Story in Her Own WordsNo ratings yet
- Raising Mentally Strong Kids: How to Combine the Power of Neuroscience with Love and Logic to Grow Confident, Kind, Responsible, and Resilient Children and Young AdultsFrom EverandRaising Mentally Strong Kids: How to Combine the Power of Neuroscience with Love and Logic to Grow Confident, Kind, Responsible, and Resilient Children and Young AdultsRating: 5 out of 5 stars5/5 (1)
- Summary: Outlive: The Science and Art of Longevity by Peter Attia MD, With Bill Gifford: Key Takeaways, Summary & AnalysisFrom EverandSummary: Outlive: The Science and Art of Longevity by Peter Attia MD, With Bill Gifford: Key Takeaways, Summary & AnalysisRating: 4.5 out of 5 stars4.5/5 (42)
- Think This, Not That: 12 Mindshifts to Breakthrough Limiting Beliefs and Become Who You Were Born to BeFrom EverandThink This, Not That: 12 Mindshifts to Breakthrough Limiting Beliefs and Become Who You Were Born to BeNo ratings yet
- Raising Good Humans: A Mindful Guide to Breaking the Cycle of Reactive Parenting and Raising Kind, Confident KidsFrom EverandRaising Good Humans: A Mindful Guide to Breaking the Cycle of Reactive Parenting and Raising Kind, Confident KidsRating: 4.5 out of 5 stars4.5/5 (169)
- Why We Die: The New Science of Aging and the Quest for ImmortalityFrom EverandWhy We Die: The New Science of Aging and the Quest for ImmortalityRating: 4 out of 5 stars4/5 (3)
- Summary: The Psychology of Money: Timeless Lessons on Wealth, Greed, and Happiness by Morgan Housel: Key Takeaways, Summary & Analysis IncludedFrom EverandSummary: The Psychology of Money: Timeless Lessons on Wealth, Greed, and Happiness by Morgan Housel: Key Takeaways, Summary & Analysis IncludedRating: 5 out of 5 stars5/5 (78)
- The Obesity Code: Unlocking the Secrets of Weight LossFrom EverandThe Obesity Code: Unlocking the Secrets of Weight LossRating: 5 out of 5 stars5/5 (4)
- The Body Keeps the Score by Bessel Van der Kolk, M.D. - Book Summary: Brain, Mind, and Body in the Healing of TraumaFrom EverandThe Body Keeps the Score by Bessel Van der Kolk, M.D. - Book Summary: Brain, Mind, and Body in the Healing of TraumaRating: 4.5 out of 5 stars4.5/5 (266)
- The Ultimate Guide To Memory Improvement TechniquesFrom EverandThe Ultimate Guide To Memory Improvement TechniquesRating: 5 out of 5 stars5/5 (34)
- Techniques Exercises And Tricks For Memory ImprovementFrom EverandTechniques Exercises And Tricks For Memory ImprovementRating: 4.5 out of 5 stars4.5/5 (40)
- The Ritual Effect: From Habit to Ritual, Harness the Surprising Power of Everyday ActionsFrom EverandThe Ritual Effect: From Habit to Ritual, Harness the Surprising Power of Everyday ActionsRating: 3.5 out of 5 stars3.5/5 (3)
- Summary: How to Be an Adult in Relationships: The Five Keys to Mindful Loving by David Richo: Key Takeaways, Summary & Analysis IncludedFrom EverandSummary: How to Be an Adult in Relationships: The Five Keys to Mindful Loving by David Richo: Key Takeaways, Summary & Analysis IncludedRating: 4 out of 5 stars4/5 (11)
- Mindset by Carol S. Dweck - Book Summary: The New Psychology of SuccessFrom EverandMindset by Carol S. Dweck - Book Summary: The New Psychology of SuccessRating: 4.5 out of 5 stars4.5/5 (328)
- Cult, A Love Story: Ten Years Inside a Canadian Cult and the Subsequent Long Road of RecoveryFrom EverandCult, A Love Story: Ten Years Inside a Canadian Cult and the Subsequent Long Road of RecoveryRating: 4 out of 5 stars4/5 (44)
- Dark Psychology & Manipulation: Discover How To Analyze People and Master Human Behaviour Using Emotional Influence Techniques, Body Language Secrets, Covert NLP, Speed Reading, and Hypnosis.From EverandDark Psychology & Manipulation: Discover How To Analyze People and Master Human Behaviour Using Emotional Influence Techniques, Body Language Secrets, Covert NLP, Speed Reading, and Hypnosis.Rating: 4.5 out of 5 stars4.5/5 (110)
- When the Body Says No by Gabor Maté: Key Takeaways, Summary & AnalysisFrom EverandWhen the Body Says No by Gabor Maté: Key Takeaways, Summary & AnalysisRating: 3.5 out of 5 stars3.5/5 (2)
- Summary: Limitless: Upgrade Your Brain, Learn Anything Faster, and Unlock Your Exceptional Life By Jim Kwik: Key Takeaways, Summary and AnalysisFrom EverandSummary: Limitless: Upgrade Your Brain, Learn Anything Faster, and Unlock Your Exceptional Life By Jim Kwik: Key Takeaways, Summary and AnalysisRating: 5 out of 5 stars5/5 (8)
- 12 Rules for Life by Jordan B. Peterson - Book Summary: An Antidote to ChaosFrom Everand12 Rules for Life by Jordan B. Peterson - Book Summary: An Antidote to ChaosRating: 4.5 out of 5 stars4.5/5 (207)
- The Garden Within: Where the War with Your Emotions Ends and Your Most Powerful Life BeginsFrom EverandThe Garden Within: Where the War with Your Emotions Ends and Your Most Powerful Life BeginsNo ratings yet
- The Happiness Trap: How to Stop Struggling and Start LivingFrom EverandThe Happiness Trap: How to Stop Struggling and Start LivingRating: 4 out of 5 stars4/5 (1)