Professional Documents
Culture Documents
Fly Memory: A Mushroom Body Story in Parts: Dispatch R855
Uploaded by
Marykate's English CoachingOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Fly Memory: A Mushroom Body Story in Parts: Dispatch R855
Uploaded by
Marykate's English CoachingCopyright:
Available Formats
Dispatch
R855
Fly Memory: A Mushroom Body Story function in the three mushroom
body subsystems (the ab, a0 b0 , and g
in Parts lobes) by using distinct combinations
of Gal4 drivers to rescue rutabaga
mutants — by driving wild-type gene
Recent studies on the compartmentalization of fly mushroom bodies show that
expression from a Gal4-controlled
learning and memory in Drosophila are not as simple as might be expected for
upstream activating sequence (UAS)
an organism with such a tiny brain.
in particular sites defined by the
regulatory elements that control where
Bruno van Swinderen the ab, a0 b0 and g lobes. Such a division GAL4 is expressed in the fly — and then
into distinct lobes (Figure 1B) tested these different classes of flies
Aristotle was notorious for categorizing suggested the segregation of distinct either immediately after training (short-
the world into parts, such as the ‘‘three functions in the mushroom body, likely term memory, STM), or 3 hours after
kinds of virtue’’ or the ‘‘four species of pertaining to the processing of odors. training (middle-term memory, MTM),
movement’’. Researchers investigating In several landmark studies spanning or 24 hours after training (long-term
brain functions such as learning a decade, Martin Heisenberg and memory, LTM).
and memory often seem to follow colleagues established that the The results of these experiments
this didactic yet effective approach. Drosophila mushroom bodies are confirm the complexity of our current
Thus, we have different phases of necessary for olfactory learning in understanding of memory formation
memory — short-term, middle-term the T-maze (reviewed in [3]). This in the fly: during training, a short-lived,
and long-term — and we try to map suggested that mushroom body rutabaga-dependent memory
these onto different structures in the intrinsic neurons act as the single (presumably involving G-protein and
brain. A study by Blum et al. [1], ‘container’ for memory storage, akin cAMP signalling) is formed in the g
published recently in Current Biology, to the simple sensory-motor system of lobes [9], while a distinct coincidence
has done this for the fruit fly Drosophila the mollusc Aplysia [4]. Following these system (possibly also involving
melanogaster. Since its beginnings initial insights into mushroom bodies, G-protein signalling [6]) originates in
three decades ago, research on a whole host of tools have been used to the a0 b0 lobes [8]. Memory is
learning and memory in Drosophila has detect and dissect memory traces in consolidated in parallel in the ab lobes
faithfully followed the Aristotelian the fly brain. There have been calcium- for longer-term effects that again
tradition of defining the component imaging studies [5,6], and studies in require rutabaga (Figure 1C). The
parts of memory. The first mutagenesis which the neurons in mushroom body recental finding of Blum et al.’s [1]
studies identified genes controlling substructures were transiently silenced study is that STM and LTM are
different phases of memory acquisition during specific phases of the functionally independent and require
and consolidation (reviewed in [2]). experiment [7,8], and ‘rescue’ different neural circuits. Thus, more
Notably, rutabaga, which encodes experiments where rutabaga function than two decades after the mushroom
a calcium-dependent adenylyl cyclase, was restored in the mushroom body bodies were first suggested as the
was found to modulate short-term lobes while left defective in the rest of location where olfactory memory is
memory and was eventually proposed the animal [9,10]. What emerged from formed and stored, it has become clear
as a site of coincidence–detection in these experiments was a complex, that the mushroom bodies are far from
neurons, where intracellular signals and at times even contradictory the equivalent of a molluscan sensory-
induced by the conditioned stimulus picture, in which both intrinsic and motor synapse. Instead, there are
(CS), such as an odor, and the extrinsic components of various parallel traces formed in different
unconditioned stimulus (US), such mushroom body subsystems were neurons at different times (some
as an electric shock, coincide shown to be necessary or sufficient requiring rutabaga, some not) and
biochemically to alter the properties for different phases of memory these processes seem to interact
of a neuron, thereby laying down formation and consolidation. dynamically with various extrinsic
a memory trace. But where in the fly Currently, the most consistent story processes [8,12,13]. Aristotle would
brain is the enzyme encoded by suggests that immediate odor be confused.
rutabaga doing this? memories require the g lobes of the Interestingly, a parallel rutabaga
If Aristotle had been able to see the mushroom bodies, while longer-lasting rescue story is unfolding for visual
internal structure of the fly brain, he memory is consolidated in ab lobes. learning in the fly. For simple visual
would probably have claimed that Memory consolidation in the ab lobes memories (involving single tethered
the mushroom bodies carry out an probably requires interactions among flies in flight arenas), the mushroom
important function that is divided into the lobes as well as between a0 b0 bodies appear to be dispensible.
three parts (Figure 1A). The mushroom neurons and mushroom body-extrinsic Instead, rutabaga function is required
bodies are paired structures composed cells, thereby making all three in the central complex, specifically in
of around 2500 neurons each that mushroom body lobes necessary for substructures of the central complex
receive synaptic input about odors via some aspect of olfactory memory called the ‘fan-shaped body’ [14] and
their ‘calyx’ (mushroom cap) near the [5,6,8,9,11,12]. To tackle this story the ‘ellipsoid body’ [15] (Figure 1D).
top of a fly’s head, and then project from another angle by systematically Recent work by Pan et al. [15] has
down and front along a ‘peduncle’ addressing where in the mushroom shown that rutabaga function must be
(mushroom stalk) before projecting into bodies rutabaga action is sufficient, restored in both the fan-shaped body
three different directions giving rise to Blum et al. [1] investigated rutabaga and ellipsoid body simultaneously to
Current Biology Vol 19 No 18
R856
required for attention-like behavior.
A B calyx-peduncle γ lobes: STM
The Drosophila mushroom bodies Whether the over-training required to
αβ lobes: LTM α'β’ lobes: STM-MTM produce olfactory LTM in the ab lobes
of mushroom bodies involves a loss of
α
α' behavioral flexibility remains an open
question. However, cumulative data
from multiple labs using different
β techniques now all agree that the
ab lobes are where we should look
β' for more permanent synaptic
γ
modifications underlying LTM
(or motor learning) in the fly model.
It is unlikely that the lobes of the
C D visual context / attention? mushroom bodies or central complex
rutabaga-dependent olfactory memory rutabaga-dependent visual memory act merely as containers storing
different kinds of memories. Instead,
most studies using the now classic
strategy of rescuing rutabaga defects
FB in the fly [1,9,10,14] suggest that more
EB dynamic measuring tools will be
required to further understand the
nature of memory in this tiny brain.
Ultimately, a thorough understanding
Current Biology
of fly memory will require insight into
neuronal events on much shorter time
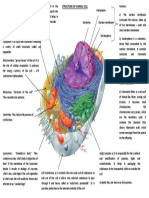
Figure 1. Memory in the Drosophila brain.
scales, such as has been shown in the
(A) Location of the paired mushroom bodies (mushroom body) in the fly brain. (B) Mushroom
mushroom body of the locust where
body parts and functions associated with olfactory learning. STM, short-term memory; MTM, local oscillations control spike-timing-
middle-term memory; LTM, long-term memory. An ab and a g neuron are depicted. (C) The dependent plasticity in the b lobes [20].
rutabaga gene is required in the g lobes for STM and in the ab lobes for LTM. (D) rutabaga The cumulative effort by workers in the
is required in the fan-shaped body (FB) and ellipsoid body (EB) of the central complex for field seems to have taken fly learning
visual learning, but not in the mushroom body. Instead, the mushroom body seems to be and memory as far as it can go within
required for visual attention-like behavior [17,18].
the realm of Aristotelian thinking. The
next step is to explain the apparently
rescue the visual learning defects of visual and olfactory processes in the dynamic interactions among the parts
a rutabaga null mutation. This result mushroom bodies must be explained, [8,12], which will probably require
suggests that there exists a dynamic even if the final repositories of better imaging and electrophysiology.
interaction between the fan-shaped memories for either modality might
body and the ellipsoid body for laying be distinct. It remains possible References
down visual memories, just as the work that the Gal4 strategy used to 1. Blum, A., Li, W., Cressy, M., and Dubnau, J.
(2009). Short and long-term memory in
by Blum et al. [1] and others [8,12] compartmentalize the fly brain Drosophila require cAMP in distinct neuron
suggests an interaction among provides results that are not as clear- types. Curr. Biol. 19, 1341–1350.
2. Davis, R.L. (2005). Olfactory memory formation
mushroom body lobes for odor cut as one might hope: Drosophila in Drosophila: from molecular to systems
memories. In this way, fly memory brain researchers have settled on a few neuroscience. Annu. Rev. Neurosci. 28,
increasingly resembles memory in key Gal4 strains to define each 275–302.
3. Gerber, B., Tanimoto, H., and Heisenberg, M.
higher-order animals where memory mushroom body or central complex (2004). An engram found? Evaluating the
traces are moved through time, such as subset, even though much else is often evidence from fruit flies. Curr. Opin. Neurobiol.
14, 737–744.
between the hippocampus and cortex labelled in these driver lines, and some 4. Hawkins, R.D., Greene, W., and Kandel, E.R.
in mammals. expression may just not be detectable. (1998). Classical conditioning, differential
To make matters even more A more optimistic view would conditioning, and second-order conditioning of
the Aplysia gill-withdrawal reflex in a simplified
complicated, the fly mushroom bodys suggest that the mushroom bodys and mantle organ preparation. Behav. Neurosci.
are not only restricted to processing the central complex are not simply 112, 636–645.
5. Yu, D., Akalal, D.B., and Davis, R.L. (2006).
and remembering odors. These static ‘filing cabinets’ for different kinds Drosophila alpha/beta mushroom body
structures appear to also be crucial of olfactory and visual learning, neurons form a branch-specific, long-term
for motor control, as well as for more respectively, but instead that neurons cellular memory trace after spaced olfactory
conditioning. Neuron 52, 845–855.
complex aspects of visual learning, in these structures are required for 6. Wang, Y., Mamiya, A., Chiang, A.S., and
such as context generalization [16] ongoing dynamic processes gating Zhong, Y. (2008). Imaging of an early memory
trace in the Drosophila mushroom body.
and visual attention-like behavior [17]. perception or motor learning [11]. J. Neurosci. 28, 4368–4376.
Indeed, the mushroom bodies are Indeed, recent flight arena work by 7. Dubnau, J., Grady, L., Kitamoto, T., and
required for restoration of visual Björn Brembs [19] suggests that the Tully, T. (2001). Disruption of neurotransmission
in Drosophila mushroom body blocks retrieval
attention defects in dunce mutants [18] mushroom bodies regulate habit but not acquisition of memory. Nature 411,
(the dunce gene has been shown to formation in Drosophila by suppressing 476–480.
8. Krashes, M.J., Keene, A.C., Leung, B.,
act with rutabaga to modulate cAMP motor learning and thus promoting Armstrong, J.D., and Waddell, S. (2007).
signalling [2]). Somehow, the overlap of behavioral flexibility, as would be Sequential use of mushroom body neuron
Dispatch
R857
subsets during Drosophila odor memory between aversive and appetitive olfactory 18. van Swinderen, B., McCartney, A.,
processing. Neuron 53, 103–115. memories in Drosophila. J. Neurosci. 23, Kauffman, S., Flores, K., Agrawal, K.,
9. Zars, T., Fischer, M., Schulz, R., and 10495–10502. Wagner, J., and Paulk, A. (2009). Shared visual
Heisenberg, M. (2000). Localization of a short- 14. Liu, G., Seiler, H., Wen, A., Zars, T., Ito, K., attention and memory systems in the
term memory in Drosophila. Science 288, Wolf, R., Heisenberg, M., and Liu, L. (2006). Drosophila brain. PLoS One 4, e5989.
672–675. Distinct memory traces for two visual 19. Brembs, B. (2009). Mushroom bodies regulate
10. McGuire, S.E., Le, P.T., Osborn, A.J., features in the Drosophila brain. Nature 439, habit formation in Drosophila. Curr. Biol. 19,
Matsumoto, K., and Davis, R.L. (2003). 551–556. 1351–1355.
Spatiotemporal rescue of memory 15. Pan, Y., Zhou, Y., Guo, C., Gong, H., Gong, Z., 20. Cassenaer, S., and Laurent, G. (2007). Hebbian
dysfunction in Drosophila. Science 302, and Liu, L. (2009). Differential roles of the fan- STDP in mushroom bodies facilitates the
1765–1768. shaped body and the ellipsoid body in synchronous flow of olfactory information in
11. Isabel, G., Pascual, A., and Preat, T. (2004). Drosophila visual pattern memory. locusts. Nature 448, 709–713.
Exclusive consolidated memory phases in Learn. Mem. 16, 289–295.
Drosophila. Science 304, 1024–1027. 16. Liu, L., Wolf, R., Ernst, R., and Heisenberg, M.
12. Keene, A.C., Krashes, M.J., Leung, B., (1999). Context generalization in Drosophila Queensland Brain Institute, The University of
Bernard, J.A., and Waddell, S. (2006). visual learning requires the mushroom bodies. Queensland, St. Lucia, Queensland, 4072
Drosophila dorsal paired medial neurons Nature 400, 753–756. Australia.
provide a general mechanism for memory 17. Xi, W., Peng, Y., Guo, J., Ye, Y., Zhang, K.,
consolidation. Curr. Biol. 16, 1524–1530. Yu, F., and Guo, A. (2008). Mushroom bodies E-mail: b.vanswinderen@uq.edu.au
13. Schwaerzel, M., Monastirioti, M., Scholz, H., modulate salience-based selective fixation
Friggi-Grelin, F., Birman, S., and Heisenberg, M. behavior in Drosophila. Eur. J. Neurosci. 27,
(2003). Dopamine and octopamine differentiate 1441–1451. DOI: 10.1016/j.cub.2009.07.064
Bacterial Evolution: Dynamic encode factors that are necessary
Genomes and the Power and, in some cases, sufficient for
pathogenesis. For example, the
of Transformation Escherichia coli locus of enterocyte
effacement (LEE) encodes a type III
secretion system required for the
attachment and effacement of these
Virulence and avirulence genes carried on large, unstable pathogenicity islands
pathogens to the intestinal lumen [2].
(PAI) strongly influence the course and fate of host–pathogen interactions. A
Similarly, the hrp/hrc cluster of the
recent study shows how one such PAI can be rapidly transferred between two
phytopathogen Pseudomonas
closely related bacteria via transformation in vivo, and how this horizontal gene
syringae encodes a type III secretion
transfer affects the fitness of the recipient strain.
system that can deliver an assortment
of over seventy type III effectors into
David S. Guttman elements, transposons, plasmids, their plant hosts [3].
and phages. While the movement of While PAIs are clearly acknowledged
A contradiction exists between our these elements is often deleterious to vary among natural isolates, the
view of organisms as extremely to the host bacterium, it can also evolutionary pressures and time
dynamic and responsive entities, result in the mobilization and required to generate this diversity is
and our common perception of these acquisition of factors critical for the typically a matter of speculation.
organisms’ genomes as static survival or success of the bacteria in Retrospective and evolutionary
structures that may change over long specific environments. Few genomic studies have been performed to
evolutionary periods, but which are elements demonstrate this as clearly map the historical record of PAI
largely isolated from the sturm und as pathogenicity islands (PAIs), which transfer and the subsequent ecological
drang of daily life. While the explosion are large, unstable chromosomal or and clinical consequences [4].
of new comparative genomic data is plasmid-borne regions encoding In vitro studies have been performed
breaking down our typological view virulence-associated or resistance to show that PAI mobilization can
of genomes, and natural genetic genes [1]. PAIs typically carry genes be induced under laboratory
variation has finally come out of the that facilitate DNA movement, such conditions. Nevertheless, it has
population genetics closet to be as integrases and transposases, are been much more difficult to study
widely appreciated as a critical flanked by direct repeats, have GC the process of acquisition, loss,
information source for clinical and contents that differ from the and transfer of PAIs in vivo. This
functional studies, there is still a genomic average, and include may be due in part to the common
disconnect between the genomic tRNAs that can act as the target belief that these events work on
variation and plasticity we see sites for DNA integration. The more a time scale incompatible with
around us and our perception of general term of ‘genomic island’ in vivo studies.
genomes as blueprints written in has been used to describe similar Important progress in understanding
permanent ink. unstable genomic regions that carry the in vivo pattern and process of
Natural selection can drive loci other than those involved in PAI transfer was made by Dawn
significant genomic changes in pathogenicity, such as those Arnold’s group at the University of
microbial populations over required for symbiosis or adaptation the West of England [5] who
dramatically short time frames. Much to specific niches. demonstrated how host–pathogen
of this genomic flux is associated PAIs are widespread among interactions can drive PAI mobilization.
with mobile elements such as insertion pathogenic bacteria and commonly The dynamics of their system are
You might also like
- Protein Synthesis GizmoDocument3 pagesProtein Synthesis Gizmoapi-522847737No ratings yet
- Bio Module 2 PhotoMasterDocument23 pagesBio Module 2 PhotoMasterh33% (3)
- Jayden Smith - Handout - Cell Organelle Review WorksheetDocument2 pagesJayden Smith - Handout - Cell Organelle Review WorksheetJayden SmithNo ratings yet
- English For Life Beginner - Can - Do - Chart PDFDocument2 pagesEnglish For Life Beginner - Can - Do - Chart PDFMarykate's English Coaching100% (1)
- LentilDocument5 pagesLentilGanpat Lal SharmaNo ratings yet
- DiafragmaDocument8 pagesDiafragmaRodrigo Diaz VqzNo ratings yet
- Worksheets Weather 1Document2 pagesWorksheets Weather 1Mónica0% (1)
- C Elegans Timbers2009Document8 pagesC Elegans Timbers2009ami501No ratings yet
- Magazine: Regeneration Lessons From The AxolotlDocument3 pagesMagazine: Regeneration Lessons From The AxolotlIPI Accounting TeamNo ratings yet
- Memoria y Plasticidad NeuronalDocument8 pagesMemoria y Plasticidad NeuronalEliezer Carvalho DantasNo ratings yet
- Lisman 2005 ADocument10 pagesLisman 2005 AnavinavinaviNo ratings yet
- Neural Control of LocomotionDocument6 pagesNeural Control of LocomotiontanviNo ratings yet
- Adam Goebel - Anatomical Evidence For EvolutionDocument3 pagesAdam Goebel - Anatomical Evidence For EvolutionAdamNo ratings yet
- Living Parts: Plant Parts Ofa CellDocument11 pagesLiving Parts: Plant Parts Ofa CellGarvNo ratings yet
- Circadian RhythmsDocument7 pagesCircadian RhythmsSeaton HarnsNo ratings yet
- PIIS096098220900918XDocument5 pagesPIIS096098220900918Xjacksparrow7425No ratings yet
- 2019 Evolution of The Chordate TelencephalonDocument16 pages2019 Evolution of The Chordate TelencephalonFabian ArenasNo ratings yet
- Learning Activity Sheet Grade 10-Science: Name: Zarzoso, Terence John J. Date: March 29, 2022 Rating/ScoreDocument4 pagesLearning Activity Sheet Grade 10-Science: Name: Zarzoso, Terence John J. Date: March 29, 2022 Rating/ScoreG03Angulo, Jamaica Bianca B.No ratings yet
- Exercise 2 General Features of Mammalian Zygote DevelopmentDocument8 pagesExercise 2 General Features of Mammalian Zygote DevelopmentCheska Mae ArnaizNo ratings yet
- Dreams RatsDocument12 pagesDreams RatsteachercarcaustoNo ratings yet
- J of Comparative Neurology - 2022 - Imam - The Brain of The Tree Pangolin Manis Tricuspis IX The Pallial TelencephalonDocument47 pagesJ of Comparative Neurology - 2022 - Imam - The Brain of The Tree Pangolin Manis Tricuspis IX The Pallial TelencephalonedyNo ratings yet
- AplasyaDocument18 pagesAplasyadanielaNo ratings yet
- Lesson Plan 2Document6 pagesLesson Plan 2Ashwarya ChandNo ratings yet
- Review LumbosacralDocument10 pagesReview LumbosacralprorocentrumNo ratings yet
- Etextbook 978 1259295645 Laboratory Manual For Holes Human Anatomy Physiology Fetal Pig Version 14th EditionDocument62 pagesEtextbook 978 1259295645 Laboratory Manual For Holes Human Anatomy Physiology Fetal Pig Version 14th Editionjerry.buttrey648100% (46)
- Ghysen 2005Document7 pagesGhysen 2005Araceli Enríquez OvandoNo ratings yet
- Exercise 1 Frog SkeletonDocument16 pagesExercise 1 Frog SkeletonMargarette IsaacNo ratings yet
- Ex. 3 Cell Types Parts and FunctionsDocument3 pagesEx. 3 Cell Types Parts and FunctionsClyde PabilonaNo ratings yet
- Mod 8 ZooDocument10 pagesMod 8 ZooLM Tricia T. DE LA CRUZNo ratings yet
- Lab 6 - Amphibian EmbryologyDocument4 pagesLab 6 - Amphibian EmbryologyAMARA ALEXIS BRACKETTNo ratings yet
- Biology - Form 4 Scheme of Work - Term 3: Topic Syllabus Objectives Activities/Labs ResourcesDocument2 pagesBiology - Form 4 Scheme of Work - Term 3: Topic Syllabus Objectives Activities/Labs ResourcesAllana HNo ratings yet
- Lobulo Antenal MelolonhidaeDocument9 pagesLobulo Antenal MelolonhidaeMile ArdilaNo ratings yet
- Naked Mole Rat SlidesDocument57 pagesNaked Mole Rat SlidesSharmila JeromeNo ratings yet
- Elife 61881 v3Document26 pagesElife 61881 v3Pegah KassraianNo ratings yet
- 1 s2.0 S1534580713001548 MainDocument14 pages1 s2.0 S1534580713001548 MainNunungTriwahyuniNo ratings yet
- Fulltext PDFDocument15 pagesFulltext PDFece142No ratings yet
- Function of Dream Sleep: Crick & Graemi M ItchisonDocument4 pagesFunction of Dream Sleep: Crick & Graemi M ItchisonTim HeisNo ratings yet
- Hagfish (Cyclostomata, Vertebrata) : Searching For The Ancestral Developmental Plan of VertebratesDocument6 pagesHagfish (Cyclostomata, Vertebrata) : Searching For The Ancestral Developmental Plan of VertebratesJ.D. NobleNo ratings yet
- Characteristics of Organisms: Assignment 8.1Document17 pagesCharacteristics of Organisms: Assignment 8.1PrabhathNo ratings yet
- Jnnpsyc00297 0079Document8 pagesJnnpsyc00297 0079EccoNo ratings yet
- Generalities: Unit 15. CerebellumDocument8 pagesGeneralities: Unit 15. CerebellumMay ZamoraNo ratings yet
- Micro e Nanoestrutura CelularDocument4 pagesMicro e Nanoestrutura CelularIsis CôrtesNo ratings yet
- Review Tubulin-Dynein System in Agellar and Ciliary MovementDocument19 pagesReview Tubulin-Dynein System in Agellar and Ciliary Movementsama EmadNo ratings yet
- The Mirror Neuron System and Its Function in HumansDocument3 pagesThe Mirror Neuron System and Its Function in HumansJúlia FerrazNo ratings yet
- Artificial Synaptic Rewiring Demonstrates That Distinct Neural Circuit Configurations Underlie Homologous BehaviorsDocument18 pagesArtificial Synaptic Rewiring Demonstrates That Distinct Neural Circuit Configurations Underlie Homologous BehaviorsRaunak PrasadNo ratings yet
- Chapter 2 MicrobioDocument39 pagesChapter 2 MicrobioEzekiel AkameNo ratings yet
- 214 The Brain From Inside Out 175: Chapter 7. Internally Organized Cell Assembly TrajectoriesDocument20 pages214 The Brain From Inside Out 175: Chapter 7. Internally Organized Cell Assembly TrajectoriesjuannnnNo ratings yet
- CBSE Class 10 Science Lab Manual - Homology and Analogy of Plants and AnimalsDocument7 pagesCBSE Class 10 Science Lab Manual - Homology and Analogy of Plants and AnimalsAryan SinghNo ratings yet
- TMP BFA2Document5 pagesTMP BFA2FrontiersNo ratings yet
- Cell Structure and VariationDocument6 pagesCell Structure and VariationZ21 Martensen AbigailNo ratings yet
- Prof. Dr.S.C.Masram Aishwarya D. Atilkar M.SC Zoology (Sem-Iii) Dept. of Zoology RTM University NagpurDocument12 pagesProf. Dr.S.C.Masram Aishwarya D. Atilkar M.SC Zoology (Sem-Iii) Dept. of Zoology RTM University Nagpurshubhamatilkar04No ratings yet
- Haemmerling's Cross-Nucleation ExperimentsDocument8 pagesHaemmerling's Cross-Nucleation ExperimentsJohn Michael WilliamsNo ratings yet
- Worksheet 4. Cell Organelles CALADODocument3 pagesWorksheet 4. Cell Organelles CALADOCalado MarvinNo ratings yet
- Subject Trimonthly Plan: Liceo de ColombiaDocument3 pagesSubject Trimonthly Plan: Liceo de ColombiaITSSebasFut7434No ratings yet
- Lec. 13 Drosophila 2 PDFDocument53 pagesLec. 13 Drosophila 2 PDFBasma RagabNo ratings yet
- Dixit Et Al 2007Document8 pagesDixit Et Al 2007Valeria FigueroaNo ratings yet
- 142 2ndJan2013BirdsongIsMusicDocument1 page142 2ndJan2013BirdsongIsMusicSubramanian AnanthanarayananNo ratings yet
- Octopuses: Quick GuideDocument2 pagesOctopuses: Quick GuideHarshitaNo ratings yet
- Workbook: Laboratory BIO32: Universiti Teknologi Mara (Uitm) Branch Sabah Campus Kota KinabaluDocument6 pagesWorkbook: Laboratory BIO32: Universiti Teknologi Mara (Uitm) Branch Sabah Campus Kota KinabaluMirza KarmilaNo ratings yet
- Spirochete Flagella and MotilityDocument16 pagesSpirochete Flagella and MotilityrehanaNo ratings yet
- Parasitology Parasitic Helminthes PDFDocument14 pagesParasitology Parasitic Helminthes PDFJuliana MendozaNo ratings yet
- Biotech JingleDocument3 pagesBiotech JinglePhol MadarangNo ratings yet
- Colors in English PDFDocument1 pageColors in English PDFMarykate's English CoachingNo ratings yet
- The Alphabet: Teaching Kids ResourceDocument2 pagesThe Alphabet: Teaching Kids ResourceMarykate's English CoachingNo ratings yet
- Legaleasy Landlaw Day1.CompressedDocument12 pagesLegaleasy Landlaw Day1.CompressedMarykate's English CoachingNo ratings yet
- Arguments-For & AgainstDocument9 pagesArguments-For & AgainstMarykate's English CoachingNo ratings yet
- Chapter 3 - Cell Structure and Function - DoneDocument7 pagesChapter 3 - Cell Structure and Function - DoneApostolos KoutsaftisNo ratings yet
- Introduction To MicrobiologyDocument54 pagesIntroduction To MicrobiologyLorelie AsisNo ratings yet
- CUT FLOWER PRODUCTION IN SRI LANKA - D.M.U.B. Dhanasekera PDFDocument4 pagesCUT FLOWER PRODUCTION IN SRI LANKA - D.M.U.B. Dhanasekera PDFtharaka888No ratings yet
- Auriscalpium VulgareDocument8 pagesAuriscalpium VulgaremarinizoNo ratings yet
- Leaves and Their FunctionDocument25 pagesLeaves and Their FunctionsrishtijrfNo ratings yet
- 01 Spontaneous Generation TheoryDocument4 pages01 Spontaneous Generation TheoryKlarence AnsayNo ratings yet
- Biology Worksheet AnswersDocument1 pageBiology Worksheet AnswersBrent Kenneth CruzNo ratings yet
- Location and Movements of Aitkanti, Tagged in SurinameDocument1 pageLocation and Movements of Aitkanti, Tagged in SurinameSuriname MirrorNo ratings yet
- Burkhardt 2001 - A Man and His MenagerieDocument9 pagesBurkhardt 2001 - A Man and His MenagerieSandra QuadrosNo ratings yet
- Mammalia LanjutanDocument28 pagesMammalia LanjutanRatih PurwaningrumNo ratings yet
- Class 9TH Biology DiversityDocument3 pagesClass 9TH Biology DiversityChirag KashyapNo ratings yet
- Culturomics of The Plant Prokaryotic Microbiome and The - 2019 - Journal of AdvaDocument13 pagesCulturomics of The Plant Prokaryotic Microbiome and The - 2019 - Journal of AdvaSarras InfoNo ratings yet
- Gram Positive Cocci Bacteriology ChartDocument2 pagesGram Positive Cocci Bacteriology ChartIsabella CeaNo ratings yet
- Module 7-Lecture 1 Microbial Biotechnology: Genetic ManipulationDocument37 pagesModule 7-Lecture 1 Microbial Biotechnology: Genetic ManipulationAakanksha RaulNo ratings yet
- Activity No. 3 The Cell As A School (Module-1)Document3 pagesActivity No. 3 The Cell As A School (Module-1)Roselie Mae GarciaNo ratings yet
- Building DNA SEDocument4 pagesBuilding DNA SERicky DunlapNo ratings yet
- ELA MAP Grade 4 PracticeDocument28 pagesELA MAP Grade 4 PracticeNicole Brown100% (1)
- Sexual and Asexual Reproduction: Program Support Notes By: © Video Education Australasia Pty LTD 2012Document12 pagesSexual and Asexual Reproduction: Program Support Notes By: © Video Education Australasia Pty LTD 2012Anonymous 7NT1wDjNo ratings yet
- Chapter 4 Nature and Composition of Crops 124136Document79 pagesChapter 4 Nature and Composition of Crops 124136S L A YNo ratings yet
- Animal BrochureDocument12 pagesAnimal BrochureWynnie RondonNo ratings yet
- Nest Architecture and Distribution of The Primitive Stingless Bee, Mourella Caerulea: Evidence For The Origin of Plebeia On The Gondwana ContinentDocument17 pagesNest Architecture and Distribution of The Primitive Stingless Bee, Mourella Caerulea: Evidence For The Origin of Plebeia On The Gondwana ContinentdphvuNo ratings yet
- Optimal Physical Parameters For Growth of Trichoderma Species at Varying PH Temperature and Agitation 2161 0517.1000127Document7 pagesOptimal Physical Parameters For Growth of Trichoderma Species at Varying PH Temperature and Agitation 2161 0517.1000127christiangrbrielNo ratings yet
- Animal Cell Mind MapDocument1 pageAnimal Cell Mind Mapsarrah asgarNo ratings yet
- 6 ThscicenceDocument4 pages6 Thscicencelearn4chatgptNo ratings yet
- In Puglia Littorina PunctataDocument6 pagesIn Puglia Littorina PunctataGabriele MacrìNo ratings yet
- Kultur Jaringan 3Document13 pagesKultur Jaringan 3Alawy FaNo ratings yet
- Marigold VarietiesDocument1 pageMarigold VarietiesDrRuby Ranjan SharmaNo ratings yet
- Socioeconomic Benefits of Shade TreesDocument18 pagesSocioeconomic Benefits of Shade TreesMulatu SimeonNo ratings yet