0% found this document useful (0 votes)
2K views2 pagesRecall Log
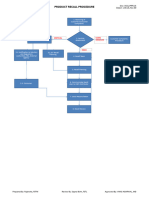
1. The document is a recall log that contains information about four product recalls between 2015-2019, including the product name, batch number, manufacturing and expiration dates, quantity released, distributed, returned/recalled, and remarks.
2. The most recent recall from October 2019 was for multiple batches of Ranidin Tablet, Ranidin Injection, and Ranidin Syrup from September 2020 due to a regulatory notice from the DGDA. The quantities involved are listed as attached.
3. The log provides details of recall reference numbers, dates initiated, reasons, and classification for four recalled pharmaceutical products over the four year period.
Uploaded by
PharmacistCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as DOCX, PDF, TXT or read online on Scribd
0% found this document useful (0 votes)
2K views2 pagesRecall Log
1. The document is a recall log that contains information about four product recalls between 2015-2019, including the product name, batch number, manufacturing and expiration dates, quantity released, distributed, returned/recalled, and remarks.
2. The most recent recall from October 2019 was for multiple batches of Ranidin Tablet, Ranidin Injection, and Ranidin Syrup from September 2020 due to a regulatory notice from the DGDA. The quantities involved are listed as attached.
3. The log provides details of recall reference numbers, dates initiated, reasons, and classification for four recalled pharmaceutical products over the four year period.
Uploaded by
PharmacistCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as DOCX, PDF, TXT or read online on Scribd
- Recall Log: Provides detailed records of product recalls including reference numbers, dates, quantities, and distribution status.