Professional Documents
Culture Documents
Lampiran: Volume Standar ×konsentrasi Sulfat Volume Total 0.25 ML × 1740 PPM 50 ML
Uploaded by
Rizky RitongaOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Lampiran: Volume Standar ×konsentrasi Sulfat Volume Total 0.25 ML × 1740 PPM 50 ML
Uploaded by
Rizky RitongaCopyright:
Available Formats
LAMPIRAN
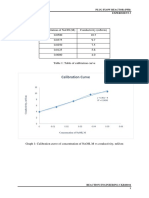
Lampiran 1 Standar kekeruhan ion sulfat (SO42-)
Kekeruhan
Volume Konsentrasi Kekeruhan
Larutan terkoreksi
(mL) (ppm) (NTU)
(NTU)
Blanko 0.00 0.00 16.00 0.00
Standar 1 0.25 8.70 21.57 5.57
Standar 2 0.50 17.4 44.20 28.20
Standar 3 0.75 26.1 59.50 43.50
Standar 4 1.00 34.8 130.33 114.33
Standar 5 1.25 43.5 158.00 142.00
Standar 6 1.50 52.2 165.67 149.67
Contoh perhitungan :
1. Kekeruhan terkoreksi = kekeruhan standar - kekeruhan blanko
= 21.57 NTU – 16.00 NTU
= 5.57 NTU
2. [SO42-] = [K2SO4] x BM K2SO4 x 1000
= 0.01 M x 174 g/mol x 1000
= 1740 ppm
Volume standar ×konsentrasi sulfat
3. [standar 1] =
Volume total
0.25 mL × 1740 ppm
=
50 mL
= 8.70 ppm
180.00
y = 3.3169x - 17.532
160.00 R² = 0.933
Kekeruhan terkoreksi (NTU)
140.00
120.00
100.00
80.00
60.00
40.00
20.00
0.00
-20.00 0.0 10.0 20.0 30.0 40.0 50.0 60.0
-40.00
Konsentrasi (ppm)
Lampiran 2 Kurva deret standar SO42-
Lampiran 3 Data pengukuran kadar sulfat dengan turbidimetri
[SO42-]
Kekeruhan terkoreksi [SO42-]
Larutan Kekeruhan (NTU) sebenarnya
(NTU) (ppm)
(ppm)
Sampel 1 114 98 34.82 1741
Sampel 2 114 98 34.82 1741
Sampel 3 115 99 35.12 1756
Rerata 1746
SD 8.660
Ketelitian (%) 99.50
Contoh perhitungan (sampel 1) :
1. Kekeruhan terkoreksi = kekeruhan sampel 1 – kekeruhan blanko
= 114 NTU – 16 NTU
= 98 NTU
2. Konsentrasi SO42- dalam sampel :
y = 3.3169x – 17.532
98 = 3.3169[SO42-] – 17.532
98 + 17.532 = 3.3169[SO42-]
[SO42-] = 34.82 ppm
3. [SO42-]sebenarnya = [SO42-]sampel 1 × FP
= 34.82 ppm × 50
= 1741 ppm
4. Rerata [SO42-]
∑ni=1 xi (1741 + 1741 + 1756)
Rerata = = = 1746 NTU
n 3
5. Standar deviasi
∑n
i=1(xi −xrerata )
2 (1741−1746)2 +(1741−1746)2 +(1756−1746)2
SD = √ =√
n−1 3−1
= 8.660
6. Ketelitian
SD 8.660
Ketelitian = |1 − rerata| × 100% = |1 − | × 100% = 99.50 %
1746
You might also like
- Compound Forming Extractants, Solvating Solvents and Inert Solvents: Iupac Chemical Data SeriesFrom EverandCompound Forming Extractants, Solvating Solvents and Inert Solvents: Iupac Chemical Data SeriesNo ratings yet
- C H O N P: PST Aeration Tank Final ClarifierDocument1 pageC H O N P: PST Aeration Tank Final ClarifierKadir EraslanNo ratings yet
- Determine Sulfate Concentration Using Standard Solutions and Turbidity MeasurementsDocument3 pagesDetermine Sulfate Concentration Using Standard Solutions and Turbidity MeasurementsAR AliNo ratings yet
- Gas Natural1Document12 pagesGas Natural1ISRAEL RODRIGUEZNo ratings yet
- Ion ChromatographyDocument9 pagesIon ChromatographyOm PhileNo ratings yet
- Sizing a Reciprocating Compressor for Natural Gas ServiceDocument6 pagesSizing a Reciprocating Compressor for Natural Gas ServiceNikhil Ashok Badgu100% (1)
- kg/cm2 M TN TN.M kg/cm2 M TN TN.M TN TN.M kg/cm2 M TN TN Tn/m3 M TN TN TN TN kg/m2 M M M MDocument4 pageskg/cm2 M TN TN.M kg/cm2 M TN TN.M TN TN.M kg/cm2 M TN TN Tn/m3 M TN TN TN TN kg/m2 M M M Mproyeccion social albañileriaNo ratings yet
- Titrration of Manganese Sulfate by Photometric EDTA TitrationDocument2 pagesTitrration of Manganese Sulfate by Photometric EDTA TitrationKeila ChavesNo ratings yet
- MoistureDocument9 pagesMoistureAshutosh MasihNo ratings yet
- RBD Analysis of Crop TreatmentsDocument9 pagesRBD Analysis of Crop TreatmentsAshutosh MasihNo ratings yet
- ZAPATA CONECTADA ExamenDocument8 pagesZAPATA CONECTADA ExamenKike ChirinosNo ratings yet
- Logsheet Boiler 10-06-2023Document696 pagesLogsheet Boiler 10-06-2023GAMING ChannelNo ratings yet
- 01 CopDocument17 pages01 CopSadia HasanNo ratings yet
- ZAPATA CONECTADA Pregunta 1Document8 pagesZAPATA CONECTADA Pregunta 1Marcelo Ramos CabreraNo ratings yet
- 8 - RTD in A CSTRDocument7 pages8 - RTD in A CSTRsansayana100% (1)
- Analisis Y Diseño de Vigas de Conexión: 0.10 Æ PrincipalDocument8 pagesAnalisis Y Diseño de Vigas de Conexión: 0.10 Æ PrincipalRONALD HUANCACHOQUE ARIASNo ratings yet
- Mercaptan Sulfur of Petroleum Products: Application NoteDocument3 pagesMercaptan Sulfur of Petroleum Products: Application NoteMiguelNo ratings yet
- Calibration Curve: Concentration of Naoh (M) Conductivity (MS/CM) 0.0500 10.7 0.0375 9.7 0.0250 7.5 0.0125 5.6 0.0000 4.0Document7 pagesCalibration Curve: Concentration of Naoh (M) Conductivity (MS/CM) 0.0500 10.7 0.0375 9.7 0.0250 7.5 0.0125 5.6 0.0000 4.0Kholidi ChooNo ratings yet
- Perhitungan Air Amonia FenatDocument3 pagesPerhitungan Air Amonia FenatGilang PermanaNo ratings yet
- Analisis Y Diseño de Vigas de Conexión: 0.10 Æ PrincipalDocument8 pagesAnalisis Y Diseño de Vigas de Conexión: 0.10 Æ PrincipalMatías F. PomiglioNo ratings yet
- Analisis Y Diseño de Vigas de Conexión: 0.10 Æ PrincipalDocument8 pagesAnalisis Y Diseño de Vigas de Conexión: 0.10 Æ PrincipalRicardo Odar GutierrezNo ratings yet
- Zapata Conectada Solucionario Del ExamenDocument13 pagesZapata Conectada Solucionario Del ExamenWaldir Riva CamascaNo ratings yet
- Reference simulation system detailsDocument17 pagesReference simulation system detailssoe0303No ratings yet
- Lab Report Experiment 1 and 2 - K2G7Document11 pagesLab Report Experiment 1 and 2 - K2G7Divagar SivaselvamNo ratings yet
- Sulphate in Mineral WaterDocument5 pagesSulphate in Mineral WaterAlfonso Pachón MarroquínNo ratings yet
- ZAPATA CONECTADA (1)Document9 pagesZAPATA CONECTADA (1)ingsfenixNo ratings yet
- Nov 2022 H2 Chemistry 9729 Paper 4 Suggested SolutionDocument20 pagesNov 2022 H2 Chemistry 9729 Paper 4 Suggested Solutionzavairling05No ratings yet
- Analisis Y Diseño de Vigas de Conexión: 0.10 Æ PrincipalDocument19 pagesAnalisis Y Diseño de Vigas de Conexión: 0.10 Æ PrincipalNilson Hugo Mendoza SalasNo ratings yet
- Data Antioksidan Mahasiswa Stfi April 2014Document11 pagesData Antioksidan Mahasiswa Stfi April 2014mevi erayaniNo ratings yet
- Cálculo Hidráulico para Aguas DansantesDocument8 pagesCálculo Hidráulico para Aguas DansantesChamillo MoralesNo ratings yet
- Solution - Colligative Properties Solutions PDFDocument25 pagesSolution - Colligative Properties Solutions PDFGOURISH AGRAWALNo ratings yet
- Diseño de Zapata Conectada.1Document9 pagesDiseño de Zapata Conectada.1Anaid PazNo ratings yet
- Analysis and design of connection beamsDocument9 pagesAnalysis and design of connection beamsAnaid PazNo ratings yet
- Analisis Y Diseño de Vigas de Conexión: 0.10 Æ PrincipalDocument9 pagesAnalisis Y Diseño de Vigas de Conexión: 0.10 Æ PrincipalArthuro TrNo ratings yet
- Analysis and design of connection beamsDocument9 pagesAnalysis and design of connection beamsHAROLD ERIKSSON GARCIA SOSANo ratings yet
- Analysis and design of connection beamsDocument8 pagesAnalysis and design of connection beamsJulio Cesar QvNo ratings yet
- Analisis Y Diseño de Vigas de Conexión: 0.10 Æ PrincipalDocument8 pagesAnalisis Y Diseño de Vigas de Conexión: 0.10 Æ PrincipalShirley Alvarado ZapataNo ratings yet
- Analisis Y Diseño de Vigas de Conexión: 0.10 Æ PrincipalDocument8 pagesAnalisis Y Diseño de Vigas de Conexión: 0.10 Æ PrincipalRoger RodriguezNo ratings yet
- Analysis and design of connection beamsDocument8 pagesAnalysis and design of connection beamsMara Rumbo Hacia DesarrolloNo ratings yet
- Analisis Y Diseño de Vigas de Conexión: 0.10 Æ PrincipalDocument8 pagesAnalisis Y Diseño de Vigas de Conexión: 0.10 Æ PrincipalNelly CondoriNo ratings yet
- Analisis Y Diseño de Vigas de Conexión: 0.10 Æ PrincipalDocument8 pagesAnalisis Y Diseño de Vigas de Conexión: 0.10 Æ PrincipalAlexandra Agurto MazuelosNo ratings yet
- Analisis Y Diseño de Vigas de Conexión: 0.10 Æ PrincipalDocument8 pagesAnalisis Y Diseño de Vigas de Conexión: 0.10 Æ PrincipalRoxana MoreNo ratings yet
- Calculo de ZapataDocument8 pagesCalculo de ZapataIsrrael MelaNo ratings yet
- Analisis Y Diseño de Vigas de Conexión: 0.10 Æ PrincipalDocument8 pagesAnalisis Y Diseño de Vigas de Conexión: 0.10 Æ PrincipalAlex MacedoNo ratings yet
- Analisis Y Diseño de Vigas de Conexión: 0.10 Æ PrincipalDocument8 pagesAnalisis Y Diseño de Vigas de Conexión: 0.10 Æ PrincipalRony Adolfo Salluca AñamuroNo ratings yet
- Analisis Y Diseño de Vigas de Conexión: 0.10 Æ PrincipalDocument8 pagesAnalisis Y Diseño de Vigas de Conexión: 0.10 Æ PrincipalPERCI IVAN VASQUEZ ROJASNo ratings yet
- Calculo de Zapata ImDocument8 pagesCalculo de Zapata ImIsrrael MelaNo ratings yet
- Analisis Y Diseño de Vigas de Conexión: 0.10 Æ PrincipalDocument8 pagesAnalisis Y Diseño de Vigas de Conexión: 0.10 Æ PrincipalOmar Jimenez AyalaNo ratings yet
- Analysis and design of connection beamsDocument8 pagesAnalysis and design of connection beamsIsaú Huamani AlfaroNo ratings yet
- Analisis Y Diseño de Vigas de Conexión: 0.10 Æ PrincipalDocument8 pagesAnalisis Y Diseño de Vigas de Conexión: 0.10 Æ PrincipalLIBARDO AUGUSTO TRIGOS RAMIREZNo ratings yet
- Analysis and design of connection beamsDocument8 pagesAnalysis and design of connection beamsEliasCastrejonSangayNo ratings yet
- Analisis Y Diseño de Vigas de Conexión: 0.10 Æ PrincipalDocument8 pagesAnalisis Y Diseño de Vigas de Conexión: 0.10 Æ PrincipalJLGS13No ratings yet
- Analysis and design of connection beamsDocument8 pagesAnalysis and design of connection beamsJannet Choi SunNo ratings yet
- Analysis and design of connection beamsDocument8 pagesAnalysis and design of connection beamsIsrrael MelaNo ratings yet
- Analisis Y Diseño de Vigas de Conexión: 0.10 Æ PrincipalDocument8 pagesAnalisis Y Diseño de Vigas de Conexión: 0.10 Æ PrincipalClarita Paola Ascate MegoNo ratings yet
- Analisis Y Diseño de Vigas de Conexión: 0.10 Æ PrincipalDocument8 pagesAnalisis Y Diseño de Vigas de Conexión: 0.10 Æ PrincipalStephanyNo ratings yet
- Analisis Y Diseño de Vigas de Conexión: 0.10 Æ PrincipalDocument8 pagesAnalisis Y Diseño de Vigas de Conexión: 0.10 Æ PrincipalIsaú Huamani AlfaroNo ratings yet
- Analisis Y Diseño de Vigas de Conexión: 0.10 Æ PrincipalDocument8 pagesAnalisis Y Diseño de Vigas de Conexión: 0.10 Æ PrincipalALFREDO CURO GOMEZNo ratings yet
- Analysis and design of connection beamsDocument8 pagesAnalysis and design of connection beamsHerbertNo ratings yet
- Analysis and design of connection beamsDocument8 pagesAnalysis and design of connection beamsRaul Valles VallesNo ratings yet
- C.R.Q. - Logarithms: F HG I KJ B GDocument1 pageC.R.Q. - Logarithms: F HG I KJ B GManoj NemaNo ratings yet
- Calculating derivatives and limits of math functionsDocument5 pagesCalculating derivatives and limits of math functionsElijah ChandruNo ratings yet
- Guide to Solving Logarithmic Equations (4037Document10 pagesGuide to Solving Logarithmic Equations (4037fizza khanNo ratings yet
- Converting Radians To Degrees and Vice VersaDocument4 pagesConverting Radians To Degrees and Vice VersaChristine Angelie GranadaNo ratings yet
- Logarithms and Exponential Functions: Skills CheckDocument7 pagesLogarithms and Exponential Functions: Skills CheckAyesha MaryamNo ratings yet
- FOUNDATION BUILDER MATHEMATICS LOGARITHM PROBLEMSDocument9 pagesFOUNDATION BUILDER MATHEMATICS LOGARITHM PROBLEMSAditya ParuiNo ratings yet
- G11 Exercises LogarithmicFunctionDocument4 pagesG11 Exercises LogarithmicFunctionBimBel PolimNo ratings yet
- Math - Logarithmic Functions and Their GraphsExercise 2-2Document18 pagesMath - Logarithmic Functions and Their GraphsExercise 2-217 JPOR Nichakorn TraipoonsinNo ratings yet
- Calculus OnlineDocument2 pagesCalculus Onlineapi-427949627No ratings yet
- Analyte, % Analyte Ratio Unit 0.5 Cvhorwitz 0.67 Cvhorwitz 2 C (-0.1505)Document4 pagesAnalyte, % Analyte Ratio Unit 0.5 Cvhorwitz 0.67 Cvhorwitz 2 C (-0.1505)Abu WildanNo ratings yet
- كراسة التمارين ١٢ ع فصل ثاني طبعة ثانيةDocument69 pagesكراسة التمارين ١٢ ع فصل ثاني طبعة ثانيةAmani EbidNo ratings yet
- Unit 1 - Ratio and ProportionDocument49 pagesUnit 1 - Ratio and ProportionVishal SinhaNo ratings yet
- Logarithm - Calculator: Find The Value of Each Logarithm Using Calculator. Round The Answer To Two Decimal PlacesDocument2 pagesLogarithm - Calculator: Find The Value of Each Logarithm Using Calculator. Round The Answer To Two Decimal PlacesOlivia Abellera CagujasNo ratings yet
- IBPS Ratios and Proportions Formulas Cracku PDFDocument14 pagesIBPS Ratios and Proportions Formulas Cracku PDFSHUBHI RAWATNo ratings yet
- WINSEM2019-20 STS5102 SS VL2019205000260 Reference Material I 18-Feb-2020 LogarithmsDocument21 pagesWINSEM2019-20 STS5102 SS VL2019205000260 Reference Material I 18-Feb-2020 LogarithmsDipali AttardeNo ratings yet
- LogarithmsDocument2 pagesLogarithmsAnikethDeltaFROST CODNo ratings yet
- Quantitative Ability Handout: Ref: QAHO1002226Document2 pagesQuantitative Ability Handout: Ref: QAHO1002226Nishu GanvirNo ratings yet
- LogarithmDocument3 pagesLogarithmsupercell.mail.helpshift.sharingNo ratings yet
- Log EquationsDocument26 pagesLog EquationsSourabh ChopraNo ratings yet
- Day 21 Ages - CeDocument8 pagesDay 21 Ages - CeyogeshNo ratings yet
- SL 2.8-2.10 Exponents and LogarithmsDocument26 pagesSL 2.8-2.10 Exponents and LogarithmsZeynep Aysu İskenderNo ratings yet
- Honors Algebra 2 Practice Exercises SolutionsDocument3 pagesHonors Algebra 2 Practice Exercises SolutionsafhNo ratings yet
- SAT ACT LogarithmsDocument7 pagesSAT ACT LogarithmsYb Andik Adi CahyonoNo ratings yet
- Over 1 Million Digits of Pi CollectedDocument489 pagesOver 1 Million Digits of Pi CollectedAnonymous 2PLTRdvhSNo ratings yet
- FOCDocument25 pagesFOCHarry QuiambaoNo ratings yet
- Logarithms: Properties and ApplicationsDocument13 pagesLogarithms: Properties and ApplicationsChandra Vamsi Adhikari100% (1)
- Table of IntegralsDocument2 pagesTable of IntegralsJeanGallegoNo ratings yet
- Maths in Focus Worked Solutions Yr 11 Adv Ch8Document111 pagesMaths in Focus Worked Solutions Yr 11 Adv Ch8LuoNo ratings yet
- Newton Raphson Method:: Find Roots of The EquationDocument15 pagesNewton Raphson Method:: Find Roots of The Equationlaila khanNo ratings yet
- Surds Practice WorksheetDocument2 pagesSurds Practice Worksheetimaad malikNo ratings yet