Professional Documents
Culture Documents
Acute Fatty Liver of Pregnancy: Better Understanding of Pathogenesis and Earlier Clinical Recognition Results in Improved Maternal Outcomes
Uploaded by
saryindrianyOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Acute Fatty Liver of Pregnancy: Better Understanding of Pathogenesis and Earlier Clinical Recognition Results in Improved Maternal Outcomes
Uploaded by
saryindrianyCopyright:
Available Formats
Acute Fatty Liver of Pregnancy:
Better Understanding of Pathogenesis
and Earlier Clinical Recognition Results
in Improved Maternal Outcomes
Authors: Ashish Goel,1 Chin Lye Ch’ng,2 *Chundamannil E. Eapen,1
Kunissery A. Balasubramanian,3 Elwyn Elias1,4
1. Hepatology Department, Christian Medical College, Vellore, India
2. Hepatology Department, Singleton Hospital, Swansea, UK
3. Wellcome Trust Research Laboratory, Division of Gastrointestinal Sciences,
Christian Medical College, Vellore, India
4. Liver Unit, University Hospital Birmingham, Birmingham, UK
*Correspondence to eapen@cmcvellore.ac.in
Disclosure: The authors have declared no conflicts of interest.
Received: 15.01.18
Accepted: 08.03.18
Keywords: Acute fatty liver of pregnancy (AFLP), improved survival, management protocol.
Citation: EMJ Hepatol. 2018;6[1]:72-79.
Abstract
Acute fatty liver of pregnancy (AFLP) is an uncommon disorder affecting women in late pregnancy.
It is increasingly recognised as an important cause of preventable maternal mortality across the
world. The pathogenic mechanism of AFLP is now better understood; it appears that a compensated
defective fatty acid oxidation becomes overt when metabolic stressors are superimposed on the
increased energy demands of late pregnancy. The mother tends to rely more on fats as a source
of energy in late pregnancy. This phenomenon may have an evolutionary basis and may explain
why AFLP typically occurs in late pregnancy. The Swansea criteria have proven to be useful in
early diagnosis of AFLP. Attempts to simplify these criteria further have proved helpful in early
recognition of the disease. Although liver biopsy showing microvesicular steatosis of hepatocytes is
the pathologic hallmark of AFLP, it is neither necessary nor safe in the antepartum setting. Current
management strategies revolve around ensuring urgent delivery of the fetus and anticipating
and managing complications of acute liver failure. While early recognition and multidisciplinary
management have considerably improved maternal survival in AFLP, fetal outcomes remain poor.
The authors postulate a therapeutic intervention to improve fetal outcomes in this disorder.
INTRODUCTION of pregnancy (AFLP); haemolysis, elevated
liver enzymes, and low platelets (HELLP)
Pregnancy-related disorders comprise several syndrome; and pre-eclamptic liver dysfunction,
poorly recognised but important causes of liver which often results in poor maternal and fetal
dysfunction that complicate various stages outcomes.1 With timely recognition, as well as
of pregnancy. These include acute fatty liver early and aggressive management, the maternal
72 HEPATOLOGY • May 2018 EMJ EUROPEAN MEDICAL JOURNAL
mortality associated with these disorders can be transition to fat metabolism in migrating birds
mitigated. Recent advances have increased the are unknown, recent studies have focussed on
current understanding of the pathogenesis of the role of peroxisome proliferator-activated
these disorders, which has translated into better receptors in regulating migratory adiposity.13
overall management of these patients. This If these animals or birds experience a hindrance
review focusses on the most under-recognised or blockage in utilising these fat stores, they could
pregnancy-related liver disorder: AFLP.2 AFLP, be expected to become energy deficient and fall
first described in 1934, was initially termed sick during these annual periods of starvation.
(as per gross liver appearance on autopsy)
In humans, the pregnant mother relies on fat
acute yellow atrophy of the liver.3,4 AFLP is
stores as the main source of energy in late
characterised by microvesicular fatty infiltration
pregnancy.14 Why would the pregnant mother
of the hepatocytes and presents in late
rely on fats as the primary energy source as
pregnancy (late second or third trimester) with
the pregnancy advances? It is possible that fats
rapidly progressive illness.5
are the preferred energy source for the mother
to tide over the reduced dietary intake, as well
EPIDEMIOLOGY as the markedly increased energy expenditure
during labour (a situation similar to the
AFLP is a rare disease, with a single large migratory bird on a long distance flight that
prospective population-based study (229 does not eat and experiences a markedly
hospitals and 1,132,964 pregnancies in the UK) increased energy expenditure). It is also possible
estimating an incidence of 5 cases per 100,000 that the mother redirects dietary carbohydrates
pregnancies (3.8–6.5 per 100,000 pregnancies; to enhance fetal nutrition. Thus, if the pregnant
confidence interval: 95%).6 The estimated mother has some defect in metabolising or
incidence of AFLP was higher in hospital-based utilising her fat stores, she can be expected to
studies from the UK7 (114 cases per 100,000 become energy deficient in late pregnancy.15
pregnancies) and India8 (30 cases per 100,000
pregnancies). Furthermore, a retrospective AFLP is characterised by mitochondrial
study from a single hospital in India found that dysfunction causing liver failure, known as
of 285 maternal deaths (in a total of 113,755 mitochondrial hepatopathy.5 Dysfunction of
pregnancies from 1999–2011), 23 (8%) were mitochondria, the power generators of the
secondary to pregnancy-related liver disorders cell, leads to energy deficiency. In the cell,
and 17 (6%) had AFLP, histologically proven in mitochondria are the sites where fatty acid
7 patients.9 oxidation takes place to produce ATP. They
are also the site where fatty acid oxidation
and glucose metabolism merge. A rat model
PATHOGENESIS
of hepatic microvesicular steatosis (induced
by sodium valproate) led to mitochondrial
The Timing of Acute Fatty Liver structural changes and oxidant stress in
of Pregnancy: Clues from an mitochondria and lysosomes of the liver.16
Evolutionary Viewpoint
Fetal Fatty Acid Oxidation Disorders
Why does this illness occur in an otherwise are Associated with Acute Fatty Liver
healthy woman in late pregnancy? The timing
of Pregnancy in the Mother
of this disease in late pregnancy may have an
evolutionary basis. During periods of starvation, In some AFLP patients, fatty acid oxidation
the human body switches from carbohydrate to disorders (commonly defects of long chain
fat stores as an energy source.10 Fat stores are 3-hydroxy acyl coenzyme A dehydrogenase
the main and preferred energy source during [LCHAD]) in the fetus are noted. The enzymes
prolonged starvation in hibernating animals (≤100 involved in fatty acid oxidation are located on
days hibernation duration) and in migratory birds the inner mitochondrial membrane. Fetal fatty
during long distance, intercontinental, nonstop acid oxidation disorders are inherited in an
flights, which can cover 3,000–4,000 km.11,12 autosomal recessive way with both parents
While the specific mechanisms regulating the being simple heterozygotes.
Creative Commons Attribution-Non Commercial 4.0 May 2018 • HEPATOLOGY 73
Compensated defect in mitochondrial
fatty acid oxidation in women
Endogenous factors* Energy switch occuring in late pregnancy Exogenous factors†
Overt defect in fatty acid oxidation
Increase in fatty acids and metabolites Oxidative stress in placenta
in placenta and maternal blood and maternal blood
Microvesicular steatosis and apoptosis of hepatocytes
Acute liver failure in the mother
Figure 1: The authors' current understanding of the pathogenesis of acute fatty liver of pregnancy.
There is an unmasking of a hitherto compensated defective fatty oxidation in women as a result of increased reliance
on fats as an energy source during late pregnancy (energy switch). This is also precipitated by endogenous factors
and/or exogenous factors.
*Fetus being homozygously defective in fatty acid oxidation or coexisting HELLP syndrome or pre-eclampsia;
†For example, drug and intercurrent infection.
HELLP: haemolysis, elevated liver enzymes, and low platelets.
In a study comparing 50 infants with fatty acid carnitine may be involved in pathogenesis
oxidation disorders to 1,250 healthy control of AFLP in these situations.19 However, other
infants, maternal liver diseases (AFLP, HELLP reports have shown a lack of association
syndrome, or pre-eclamptic liver dysfunction) between fetal fatty acid oxidation disorders and
were noted in 16.0% of fatty acid oxidation maternal liver diseases.20,21
defect pregnancies compared with 0.9%
observed in the control group (odds ratio: 20.4; The Placenta as a Driver of
95% confidence interval: 7.8–53.2). Different Pathogenesis of Acute Fatty
types of fetal fatty acid oxidation defects were Liver of Pregnancy
associated with maternal liver disease. While
infants with LCHAD defects were 50-times The placenta shares a similar genetic profile to
more likely to be associated with maternal the fetus. Mitochondrial enzymes are expressed
liver disease than controls, infants with short in the placenta. A dramatic improvement in the
and medium chain fatty acid oxidation defects health of the mother with AFLP often occurs
were 12-times more likely to be associated with after delivery of the baby and the placenta.
maternal liver disease.17 Additionally, an analysis This observation led to studies on the placenta
of 63 pregnancies in 18 mothers with a total in AFLP patients.22 Raised levels of free fatty
of 28 LCHAD-deficient children noted AFLP, acids were seen in the placenta and serum
HELLP syndrome, and pre-eclampsia in 31%, of AFLP patients compared to controls;
and intrahepatic cholestasis in 10% of arachidonic acid and palmitic acid levels were
pregnancies, but in none of the pregnancies higher in the placenta and serum of AFLP
with a healthy fetus.18 Accumulation of acyl patients while oleic acid and myristic acid
74 HEPATOLOGY • May 2018 EMJ EUROPEAN MEDICAL JOURNAL
levels were higher in the placenta of AFLP of AFLP in a suspected patient. Thus, liver
patients. Serum arachidonic acid levels were biopsy is not advisable before swiftly embarking
4-fold higher in AFLP patients (80 μm/mL) on management.27 Post-partum liver biopsy
compared to healthy pregnant controls confirmation of AFLP may help in patient
(20 μm/mL). Mitochondrial dysfunction and counselling (regarding further pregnancies) and
increased oxidative and nitrosative stress were individualising management of the child. The
also noted in the placenta and serum of AFLP transjugular route provides a safe access point
mothers compared to controls.23 for liver biopsy in patients with coagulopathy.28
The microvesicular fatty infiltration of the
Arachidonic acid, at the concentration seen in hepatocytes is noted to rapidly resolve after
serum of AFLP patients (80 μm/mL), induced delivery and thus liver biopsy, if undertaken, is
mitochondrial dysfunction, oxidative stress in preferably done within 4 days after delivery.27
mitochondria, apoptosis (without necrosis), The presentation of symptoms and signs and
and fat deposition, suggestive of microvesicular basic lab investigations form the basis of the
steatosis, in Chang liver cell lines.23 Figure 1 Swansea diagnostic criteria for AFLP.
summarises the authors’ current understanding
of pathogenesis of AFLP.
DIAGNOSIS
CLINICAL FEATURES Realising the lack of diagnostic criteria for AFLP,
AND INVESTIGATIONS Ch’ng et al.,7 based on retrospective analysis
of multiple case series, proposed the Swansea
AFLP is typically limited to late pregnancy and criteria for AFLP diagnosis. These criteria
the patient usually presents with vomiting, are based on typical presentation, absence
abdominal pain, and mild jaundice;24 symptoms of an alternate explanation, and laboratory
of polydipsia and polyuria can be present but parameters noted in patients with AFLP.7 These
are rarely seen. criteria were later prospectively validated
Though it is more common in primigravida, against a clinical diagnosis by Knight et al.6
AFLP can occur in multiparous females with a In a retrospective study of 24 patients with
history of prior uneventful pregnancies.2 The suspected pregnancy-related liver disease,
clinical course often progresses rapidly downhill, the Swansea criteria were validated against
with eventual occurrence of encephalopathy, the gold standard for diagnosis, i.e., diffuse or
hypoglycaemia, and/or ascites. Rare complications perivenular microvesicular hepatic steatosis on
include liver rupture and haemoperitoneum.2 liver biopsy, obtained in either the immediate
postnatal (n=19) or post-mortem (n=5)
At presentation, patients may or may not period.27 Thus, antenatal application of the
be clinically jaundiced with a mild-to- Swansea criteria (even without liver biopsy;
moderate increase in aminotransferases. i.e., presence of 6 of the 13 remaining criteria)
Coagulopathy (prolongation of prothrombin is useful in diagnosing clinical AFLP and also
time and/or hypofibrinogenaemia), renal predicts presence of microvesicular steatosis
failure, hyperuricaemia, hypoglycaemia, and on liver biopsy.29 These diagnostic criteria have
leukocytosis are commonly observed at increased the ability to suspect AFLP (as they
presentation or over the next few days. have very high negative predictive value) and to
Ultrasound scan showing fatty acid infiltration institute early management of these patients.30-32
of the liver is neither sensitive nor specific for
the diagnosis of AFLP.24 The simplified criteria to diagnose AFLP states
that the disease should be suspected in all
The most specific, gold standard investigation women presenting in the late second or third
to reach the diagnosis is liver biopsy trimester of pregnancy with unexplained acute
demonstrating diffuse or perivenular liver failure (i.e., jaundice with coagulopathy
microvesicular steatosis.25,26 Presence of often accompanied by encephalopathy and/or
coagulopathy and difficulties in performing liver hypoglycaemia).33 The time required to evaluate
biopsy in the antenatal mother are challenges other causes of liver failure has to be balanced
that impact obtaining histological confirmation against the urgency of delivery.
Creative Commons Attribution-Non Commercial 4.0 May 2018 • HEPATOLOGY 75
Liver dysfunction in second and third trimester of pregnancy
Clinical and laboratory evaluation
(liver biopsy not advisable antepartum)
Rule out other causes of acute liver failure (geographic locality specific)
Coagulopathy
(± encephalopathy ± hypoglycaemia)
AFLP is a possibility
Ensure urgent delivery (caesarean section preferred)
Adequate blood support (especially prior to delivery)
Ensure adequate dextrose/glucose intake (>2,000 kcal/day)
Broad-spectrum antibiotic prophylaxis
Manage and anticipate complications
Figure 2: Management protocol in patients suspected to have acute fatty liver of pregnancy.
AFLP: acute fatty liver of pregnancy.
Box 1: Basis for the proposed management strategy to reduce perinatal mortality in acute fatty liver of pregnancy.
Normal pregnancy
• Increased caloric demand in later stages of pregnancy.
• Switch to fats as preferred energy substrate.
Acute fatty liver of pregnancy
• Accumulation of fatty acids that have not undergone beta oxidation at the required rate.
• Inability to generate energy at the rate required to sustain a healthy pregnancy and labour.
• Energy deficiency in the fetus.
• Consumption of available glucose resulting in damaging hypoglycaemia.
Proposal for treatment
• Provide sufficient glucose or dextrose as substitute substrate to meet energy requirements of the fetus.
The cause(s) that needs to be evaluated overlap.27,37 Typical changes on liver biopsy may
depend on the aetiology of acute liver failure help in differentiating these conditions. Whether
within that geographic locality and needs to this overlap is due to the non-specific nature
be individualised for each patient (e.g., taking of the diagnostic criteria or are secondary to
into account hepatitis A and E virus infection overlap in pathogenesis is unclear and needs
in endemic areas).34,35 Clinicians evaluate for further clarification. Xiao et al.38 suggested
drug-induced liver injury (by obtaining history that HELLP syndrome is caused by endothelial
of ingestion of potentially hepatotoxic drugs), dysfunction leading to secondary thrombotic
malaria (peripheral smear examination), microangiopathy. It is likely that the initial
and acute viral hepatitis B before a diagnosis event (either AFLP or HELLP) contributes
of AFLP is entertained.3,36 additional stress that stimulates the other in a
genetically predisposed individual (Figure 1).
Overlap of Diagnosis with Other The management of disease likely remains
Pregnancy-Related Liver Disorders unaltered, and so it may not be essential to
make such a differentiation at present.
In most patients, the diagnostic criteria for AFLP
and HELLP syndrome show a considerable
76 HEPATOLOGY • May 2018 EMJ EUROPEAN MEDICAL JOURNAL
MANAGEMENT team approach, and thus early recognition and
referral to an experienced and equipped centre
is often required. Instituting early and aggressive
Prompt suspicion, early recognition, and
management protocols (Figure 2) has reduced
emergent careful delivery of the baby remain
maternal deaths at most institutions across
the cornerstones of management of patients
the world.2
with AFLP.4,39,40 Maternal survival is to be
prioritised and any delay either in recognition
of AFLP or in instituting delivery of the baby PROPOSED THERAPEUTIC
can adversely affect maternal outcome. It is INTERVENTION TO IMPROVE
important to recognise and categorise these FETAL SURVIVAL IN ACUTE
patients as seriously sick and consider the FATTY LIVER OF PREGNANCY
intensive care or high dependency unit for
disease management. The general principles In AFLP, the postulated underlying defect
guiding management in patients with acute is an inability to meet energy requirements
liver failure (e.g., intravenous mannitol infusions in the face of a) the extremely high calorie
for cerebral oedema) are also applicable for requirements of late pregnancy (additional
patients with AFLP, but these are beyond the 500 kcals per day is typically required during
scope of this article. the third trimester)48 and b) genetic defects
Constant involvement of various specialities, restricting the rate at which fatty acid
such as obstetrics, hepatology, intensive care, oxidation can proceed. The baby’s energy
anaesthesia, haematology, and neonatology, requirements cannot be met by beta oxidation
is a must for optimal care of an AFLP patient. of fatty acids, and therefore dextrose or
The mode of delivery is to be decided on a glucose supplementations are necessary to
case by case basis by an obstetrician. Vaginal counter the deficiency in substrate metabolism
delivery possibly entails a longer delivery and energy release. We propose that upon
duration, as well as possible worsening of diagnosis of AFLP, the mother should be fed
liver and/or multi-organ dysfunction, because with dextrose in amounts that provide the
added energy is needed for vaginal delivery required 2,000 calories per day or more.48 This
in an AFLP patient who is already in a state proposed intervention, aimed at improving fetal
of systemic energy deficit consequent to survival in AFLP, needs to be tested in clinical
mitochondrial hepatopathy; in comparison, studies (Box 1).
caesarean section poses an increased risk of
Care of the Baby
bleeding and anaesthesia-related complications.
Most centres prefer delivering the baby by The management of babies born to mothers
caesarean section with careful anaesthetic who have AFLP needs specialised inputs from
management.41,42 Adequate blood product a neonatologist and clinical geneticist. The
replacement to address coagulopathy is baby must be assessed for fatty acid oxidation
a prerequisite for both modes of delivery. defects.25,49 Specific fatty acid oxidation defects
Hypoglycaemia and postpartum haemorrhage (e.g., E474Q mutation in LCHAD component of
are common complications that need to be mitochondrial trifunctional protein) are noted
anticipated;43,44 additionally, the use of broad- in some populations.49,50 Some authors suggest
spectrum antibiotics as a prophylaxis should universal screening for LCHAD deficiency
also be considered. (by acyl-carnitine assay) in all children born
to mothers with AFLP,51 as LCHAD defects are
Intensive monitoring and continued
the most common identified defects.52,53
management in the complication of acute
liver failure is often required in the post- The presentation of children with fatty acid
partum period. Occasionally, patients may oxidation defects can be extremely variable,
require prolonged supportive management, from being asymptomatic to severely ill
including plasma exchange, and only rarely is with encephalopathy, cardiomyopathy, or even
liver transplant warranted.45-47 Managing AFLP sudden infant death. The symptoms tend to be
patients demands an intensive multidisciplinary precipitated by catabolic stress and all efforts
Creative Commons Attribution-Non Commercial 4.0 May 2018 • HEPATOLOGY 77
are required to mitigate this complication at challenge and improvement in management
the earliest signs.54 strategies may be required to address this
important issue.
Outcome
Future Pregnancies
Most AFLP mothers show rapid improvement
within a few days after delivery and on follow- There is a small but definite risk of recurrence
up liver functions revert to normal.55 The during subsequent pregnancies, especially if
prognosis in these patients is determined by there is a demonstrable defect in fatty acid
the severity of liver dysfunction (serum bilirubin oxidation.60,61 This should be discussed in detail
and prothrombin time), serum creatinine, and with the patient.
delay in delivery.56-58
Consequent to protocol-based urgent delivery CONCLUSION
(simplified criteria to diagnose AFLP), a steady
decline in the contribution of AFLP and other Improved understanding of the pathogenesis
pregnancy-related liver disorders to maternal of AFLP, early recognition using clinical
mortality has been reported in one centre diagnostic criteria, and aggressive management
(maternal mortality due to pregnancy-related has resulted in improvement in maternal
liver disorders decreased from 13% between mortality in many centres across the world.
1999 and 2003 to 5% between 2004 and 2011).9 Continued efforts are required to increase
Similar results have been noted across various awareness regarding this preventable cause of
studies, with the maternal mortality due to maternal death. Further focus should be directed
pregnancy related liver disorders now expected towards the improvement of fetal survival in
to be <10%.2,4,5,59 The fetal outcome remains a mothers with AFLP.
References
1. Westbrook RH et al. Pregnancy and Gastroenterol. 2007;26(Suppl 2):A83. peroxisomes. J Gastroenterol Hepatol.
liver disease. J Hepatol. 2016;64(4): 2006;21(8):1240-9.
933-45. 10. Palmiere C et al. Postmortem
biochemistry in suspected starvation- 17. Browning MF et al. Fetal fatty acid
2. Hay JE. Liver disease in pregnancy. induced ketoacidosis. J Forensic Leg oxidation defects and maternal liver
Hepatology. 2008;47(3):1067-76. Med. 2016;42:51-5. disease in pregnancy. Obstet Gynecol.
2006;107(1):115-20.
3. Goel A et al. Pregnancy-related 11. Rigano KS et al. Life in the fat
liver disorders. J Clin Exp Hepatol. lane: Seasonal regulation of insulin 18. Tyni T et al. Pregnancy complications
2014;4(2):151-62. sensitivity, food intake, and adipose are frequent in long-chain
biology in brown bears. J Comp 3-hydroxyacyl-coenzyme A
4. Liu J et al. Acute fatty liver disease of Physiol B. 2017;187(4):649-76. dehydrogenase deficiency. Am J
pregnancy: Updates in pathogenesis,
Obstet Gynecol. 1998;178(3):603-8.
diagnosis, and management. Am J 12. Jenni-Eiermann S. Energy metabolism
Gastroenterol. 2017;112(6):838-46. during endurance flight and the post- 19. Eskelin PM et al. Elevated
flight recovery phase. J Comp Physiol hydroxyacylcarnitines in a carrier
5. Eapen CE et al. Liver failure during A Neuroethol Sens Neural Behav of LCHAD deficiency during acute
pregnancy. Gut. 2008;57(1):83. Physiol. 2017;203(6-7):431-8. liver disease of pregnancy - A
6. Knight M et al.; UK Obstetric 13. Corder KR et al. Annual life-stage common feature of the pregnancy
Surveillance System. A prospective regulation of lipid metabolism and complication? Mol Genet Metab.
national study of acute fatty liver storage and association with PPARs 2010;100(2):204-6.
of pregnancy in the UK. Gut. in a migrant species: The gray catbird 20. Holub M et al. Lack of correlation
2008;57(7):951-6. (Dumetella carolinensis). J Exp Biol. between fatty acid oxidation
2016;219:3391-8.
7. Ch’ng CL et al. Prospective disorders and haemolysis, elevated
study of liver dysfunction in 14. Ghio A et al. Triglyceride metabolism liver enzymes, low platelets
pregnancy in southwest Wales. Gut. in pregnancy. Adv Clin Chem. 2011;55: (HELLP) syndrome? Acta Paediatr.
2002;51(6):876-80. Erratum in: Gut. 133-53. 2005;94(1):48-52.
2003;52(2):315.
15. Zachariah U et al. The hibernating 21. Raghupathy V et al. Absence of
8. Rathi U et al. Effect of liver disease bear-A good analogy to explain G1528C mutation in long-chain
on maternal and fetal outcome why acute fatty liver of pregnancy 3-hydroxyacyl-CoA dehydrogenase in
– A prospective study. Indian J manifests in late pregnancy. Am J four Indian patients with pregnancy-
Gastroenterol. 2007;26(2):59-63. Gastroenterol. 2018;113(2):307-8. related liver disease. Indian J
Gastroenterol. 2014;33(4):387-9.
9. Goel A et al. Contribution of 16. Natarajan SK et al. Oxidative stress
pregnancy related liver diseases to in experimental liver microvesicular 22. Natarajan SK et al. Acute fatty
maternal mortality at Vellore. Indian J steatosis: Role of mitochondria and liver of pregnancy: An update
78 HEPATOLOGY • May 2018 EMJ EUROPEAN MEDICAL JOURNAL
on mechanisms. Obstet Med. failure in pregnancy. Crit Care Clin. 48. German Nutrition Society (DGE). New
2011;4(3):99-103. 2016;32(1):61-72. reference values for energy intake.
Ann Nutr Metab. 2015;66(4):219-23.
23. Natarajan SK et al. Liver injury 37. Minakami H et al. Differentiation of
in acute fatty liver of pregnancy: acute fatty liver of pregnancy from 49. Yang Z et al. Prospective screening
Possible link to placental syndrome of hemolysis, elevated liver for pediatric mitochondrial
mitochondrial dysfunction and enzymes and low platelet counts. J trifunctional protein defects in
oxidative stress. Hepatology. 2010; Obstet Gynaecol Res. 2014;40(3): pregnancies complicated by liver
51(1):191-200. 641-9. disease. JAMA. 2002;288(17):2163-6.
24. Zhu T et al. [Screening time and 38. Xiao J et al. Expression of ADAMTS13 50. Yang Z et al. Fetal genotypes and
schedule for outpatients with acute in normal and abnormal placentae pregnancy outcomes in 35 families
fatty liver of pregnancy]. Zhong and its potential role in angiogenesis with mitochondrial trifunctional
Nan Da Xue Xue Bao Yi Xue Ban. and placenta development. protein mutations. Am J Obstet
2015;40(7):748-53. (In Chinese). Arterioscler Thromb Vasc Biol. Gynecol. 2002; 187(3):715-20.
2017;37(9):1748-56.
25. Joshi D et al. Liver disease in 51. Shekhawat PS et al. Fetal fatty acid
pregnancy. Lancet. 2010;375(9714): 39. Kamimura K et al. Advances in oxidation disorders, their effect
594-605. understanding and treating liver on maternal health and neonatal
diseases during pregnancy: A outcome: Impact of expanded
26. Rolfes DB, Ishak KG. Acute fatty liver review. World J Gastroenterol. newborn screening on their diagnosis
of pregnancy: A clinicopathologic 2015;21(17):5183-90. and management. Pediatr Res.
study of 35 cases. Hepatology. 2005;57(5 Pt 2):78R-86R.
1985;5(6): 1149-58. 40. Liu G et al. Acute fatty liver of
pregnancy: Analysis on the diagnosis 52. Bo R et al. Clinical and molecular
27. Goel A et al. How accurate are the and treatment of 15 cases. J Reprod investigation of 14 Japanese patients
Swansea criteria to diagnose acute Med. 2016;61(5-6):282-6. with complete TFP deficiency:
fatty liver of pregnancy in predicting A comparison with Caucasian cases.
hepatic microvesicular steatosis? Gut. 41. Wang HY et al. Effect of caesarean J Hum Genet. 2017;62(9):809-14.
2011;60(1):138-9. section on maternal and foetal
outcomes in acute fatty liver of 53. Ibdah JA et al. Molecular prenatal
28. Mammen T et al. Transjugular liver pregnancy: A systematic review and diagnosis in families with fetal
biopsy: A retrospective analysis of meta-analysis. Sci Rep. 2016;6:28826. mitochondrial trifunctional protein
601 cases. J Vasc Interv Radiol. 2008; mutations. J Pediatr. 2001;138(3):
19(3):351-8. 42. Qu YY et al. [Retrospective analysis 396-9.
of anesthetic and perioperative
29. Wang S et al. Noninvasive Swansea management in patients of acute 54. Anon B et al. [Acute fatty liver of
criteria are valuable alternatives fatty liver of pregnancy]. Zhonghua pregnancy and mitochondrial fatty
for diagnosing acute fatty liver of Yi Xue Za Zhi. 2017;97(24):1878-82. acid oxidation. Consequences for the
pregnancy in a Chinese population. (In Chinese). offspring]. Arch Pediatr. 2017;24(8):
J Matern Fetal Neonatal Med. 777-82. (In French).
2017;30(24):2951-5. 43. Crochemore T et al.
Thromboelastometry-guided 55. Xiong HF et al. Acute fatty liver of
30. Ilham Aldika Akbar M et al. Clinical hemostatic therapy: An efficacious pregnancy: Over six months follow-up
characteristics of acute fatty liver of approach to manage bleeding risk in study of twenty-five patients. World J
pregnancy in a tertiary Indonesian acute fatty liver of pregnancy: A case Gastroenterol. 2015;21(6):1927-31.
hospital. J Matern Fetal Neonatal report. J Med Case Rep. 2015;9:202.
Med. 2017:1-191. 56. Zhang YP et al. Acute fatty liver of
44. Goel A et al. Preliminary experience pregnancy: A retrospective analysis
31. Solanke D et al. Etiology, clinical with use of recombinant activated of 56 cases. Chin Med J (Engl). 2016;
profile, and outcome of liver disease factor VII to control postpartum 129(10):1208-14.
in pregnancy with predictors of hemorrhage in acute fatty liver of
maternal mortality: A prospective 57. Meng J et al. Prenatal predictors in
pregnancy and other pregnancy
study from Western India. Indian J postpartum recovery for acute fatty
related liver disorders. Indian J
Gastroenterol. 2016;35(6):450-8. liver of pregnancy: Experiences at a
Gastroenterol. 2013;32(4):268-71.
tertiary referral center. Arch Gynecol
32. Mishra N et al. Study of abnormal liver 45. Ding J et al. Effectiveness of Obstet. 2015;293:1185-91.
function test during pregnancy in a combining plasma exchange with
tertiary care hospital in Chhattisgarh. 58. Gao Q et al. Outcome and risk
plasma perfusion in acute fatty
J Obstet Gynaecol India. 2016;66 factors of patients with acute fatty
liver of pregnancy: A retrospective
(Suppl 1):129-35. liver of pregnancy: A multicentre
analysis. Gynecol Obstet Invest.
retrospective study. Singapore Med
33. Goel A et al. Simplified diagnostic 2015;79:97-100.
J. 2018. [Epub ahead of print].
criteria for acute fatty liver of 46. He N et al. [Retrospective study on
pregnancy. J Gastroenterol Hepatol. 59. Fesenmeier MF et al. Acute fatty
efficacy and safety of the sequential liver of pregnancy in 3 tertiary care
2010;25:A113. use of a non-bioartificial liver centers. Am J Obstet Gynecol.
34. Acharya SK et al. Etiopathogenesis of support system in the treatment of 2005;192(5):1416-9.
acute hepatic failure: Eastern versus acute fatty liver during pregnancy].
Western countries. J Gastroenterol Zhonghua Gan Zang Bing Za Zhi. 60. Visconti M et al. Recurrence of
Hepatol. 2002;17(Suppl 3):S268-73. 2015;23(10):775-7. (In Chinese). acute fatty liver of pregnancy. J Clin
Gastroenterol. 1995;21(3):243-5.
35. Puliyath G, Singh S. Leptospirosis in 47. Ringers J et al. Auxiliary or orthotopic
pregnancy. Eur J Clin Microbiol Infect liver transplantation for acute fatty 61. Bacq Y et al. [Recurrent acute fatty
Dis. 2012;31(10):2491-6. liver of pregnancy: Case series liver of pregnancy]. Gastroenterol
and review of the literature. BJOG. Clin Biol. 2007;31(12):1135-8.
36. Bacak SJ, Thornburg LL. Liver 2016;123:1394-8. (In French).
Creative Commons Attribution-Non Commercial 4.0 May 2018 • HEPATOLOGY 79
You might also like
- Obesity - Short Scientific Findings to Ameliorate the Body WeightFrom EverandObesity - Short Scientific Findings to Ameliorate the Body WeightNo ratings yet
- Maternal Cardiac Metabolism in Pregnancy: Spotlight ReviewDocument9 pagesMaternal Cardiac Metabolism in Pregnancy: Spotlight ReviewAlika MaharaniNo ratings yet
- Liver Disease in Pregnancy: J. Eileen HayDocument10 pagesLiver Disease in Pregnancy: J. Eileen HayNirmala PudakalkattiNo ratings yet
- Acute Fatty Liver in PregnancyDocument2 pagesAcute Fatty Liver in PregnancyyudhistirakusNo ratings yet
- Acute Fatty Liver in PregnancyDocument2 pagesAcute Fatty Liver in PregnancyyudhistirakusNo ratings yet
- Fatty Liver in Pregnancy - StatPearls - NCBI BookshelfDocument6 pagesFatty Liver in Pregnancy - StatPearls - NCBI BookshelfAndres Pimentel AlvarezNo ratings yet
- Geenes 2016Document9 pagesGeenes 2016Christian YzaguirreNo ratings yet
- Kwashiorkor MarasmoDocument16 pagesKwashiorkor MarasmoW Antonio Muñoz ChNo ratings yet
- Official Reprint From Uptodate ©2017 Uptodate, Inc. And/Or Its Affiliates. All Rights ReservedDocument22 pagesOfficial Reprint From Uptodate ©2017 Uptodate, Inc. And/Or Its Affiliates. All Rights ReservedEnrico HerviantoNo ratings yet
- Acute Fatty Liver of Pregnancy: Pathophysiology, Anesthetic Implications, and Obstetrical ManagementDocument16 pagesAcute Fatty Liver of Pregnancy: Pathophysiology, Anesthetic Implications, and Obstetrical Managementdr_syakbanNo ratings yet
- Acute Fatty Liver of Pregnancy: An Update On Pathogenesis and Clinical ImplicationsDocument8 pagesAcute Fatty Liver of Pregnancy: An Update On Pathogenesis and Clinical ImplicationsRembulanAyuNiendhitaPutriNo ratings yet
- Acute Fatty Liver of Pregnancy.23Document12 pagesAcute Fatty Liver of Pregnancy.23AbrilNo ratings yet
- Maternal Obesity Causes Fetal Hypothalamic Insulin Resistance and Disrupts Development of Hypothalamic Feeding PathwaysDocument10 pagesMaternal Obesity Causes Fetal Hypothalamic Insulin Resistance and Disrupts Development of Hypothalamic Feeding PathwaysAgustin LopezNo ratings yet
- Doença Hepática Na GravidezDocument12 pagesDoença Hepática Na GravidezRodrigo MaiaNo ratings yet
- Prenancy Tool Revise March 7Document7 pagesPrenancy Tool Revise March 7BamwinataNo ratings yet
- Metabolism in Normal PregnancyDocument10 pagesMetabolism in Normal Pregnancywilliam pinillaNo ratings yet
- Postgrad Med J 2010 Mackillop 160 4Document6 pagesPostgrad Med J 2010 Mackillop 160 4Gonzalo MendozaNo ratings yet
- Practical Dietary Management of Protein Energy Malnutrition in Young Children With Cow's Milk Protein AllergyDocument8 pagesPractical Dietary Management of Protein Energy Malnutrition in Young Children With Cow's Milk Protein AllergyCarlos CuadrosNo ratings yet
- No 3Document16 pagesNo 3Bian DaraNo ratings yet
- 1 s2.0 S0955286317303868 MainDocument14 pages1 s2.0 S0955286317303868 MainAdib FraNo ratings yet
- TOG Intrahepatic Cholestasis of PregnancyDocument9 pagesTOG Intrahepatic Cholestasis of PregnancyMarNo ratings yet
- Clinical Approach To Inborn Errors of Metabolism - Presenting in The Newborn PeriodDocument7 pagesClinical Approach To Inborn Errors of Metabolism - Presenting in The Newborn Periodmaxime wotolNo ratings yet
- Maternal High-Fat Diet Induces Obesity and Adrenal and Thyroid Dysfunction in Male Rat Offspring at WeaningDocument16 pagesMaternal High-Fat Diet Induces Obesity and Adrenal and Thyroid Dysfunction in Male Rat Offspring at WeaningDeaz Fazzaura PutriNo ratings yet
- Fat Malabsorption in Critical IllnessDocument6 pagesFat Malabsorption in Critical Illnesslakshminivas PingaliNo ratings yet
- Diet-Induced Obesity in Female Mice Leads To Offspring Hyperphagia, Adiposity, Hypertension, and Insulin ResistanceDocument11 pagesDiet-Induced Obesity in Female Mice Leads To Offspring Hyperphagia, Adiposity, Hypertension, and Insulin ResistanceOris WicaksonoNo ratings yet
- The Diagnosis and Management of Acute Fatty Liver of PregnancyDocument7 pagesThe Diagnosis and Management of Acute Fatty Liver of PregnancyMuhammad Fariz SalehNo ratings yet
- ApolipoproteinDocument12 pagesApolipoproteinShilpa.PNo ratings yet
- Maternal Carbohydrate Intake and Pregnancy OutcomeDocument6 pagesMaternal Carbohydrate Intake and Pregnancy OutcomeSofía Simpértigue CubillosNo ratings yet
- Parenteral Nutrition Patients: ObstetricDocument14 pagesParenteral Nutrition Patients: ObstetricAripinSyarifudinNo ratings yet
- Resistencia A La Insulina Madre y FetoDocument10 pagesResistencia A La Insulina Madre y FetoNorma AlvarezNo ratings yet
- F A C N - M: Rom The Merican Ollege of Urse IdwivesDocument8 pagesF A C N - M: Rom The Merican Ollege of Urse Idwivesbellia loranthifoliaNo ratings yet
- Glucose Intolerance and Insulin Resistance: Relevance in PreeclampsiaDocument14 pagesGlucose Intolerance and Insulin Resistance: Relevance in PreeclampsiaEgi NabilaNo ratings yet
- Cancer Anorexia and CachexiaDocument5 pagesCancer Anorexia and CachexiaWildan Satrio WemindraNo ratings yet
- Pregnancy-Related Liver Disorders: Seminar Journal of Clinical and Experimental HepatologyDocument12 pagesPregnancy-Related Liver Disorders: Seminar Journal of Clinical and Experimental HepatologysaryindrianyNo ratings yet
- Maternal C Reactive Protein and Developmental ProgDocument5 pagesMaternal C Reactive Protein and Developmental ProgIriawan Indra PutraNo ratings yet
- FattyliverinpregnancyDocument8 pagesFattyliverinpregnancyJulieta Urano DelgadoNo ratings yet
- NutritioninPregnancyDocument7 pagesNutritioninPregnancyBamwinataNo ratings yet
- Protein Metabolism in Pregnancy: Satish C KalhanDocument7 pagesProtein Metabolism in Pregnancy: Satish C KalhanAdib FraNo ratings yet
- Nutrition and Reproduction in Women: The ESHRE Capri Workshop GroupDocument15 pagesNutrition and Reproduction in Women: The ESHRE Capri Workshop GroupmamaazkaNo ratings yet
- Backgroun 1Document3 pagesBackgroun 1Idi Nagan RayaNo ratings yet
- Prediction of Preeclampsia PDFDocument19 pagesPrediction of Preeclampsia PDFAlejandro FrancoNo ratings yet
- Neonatal: HypoglycemiaDocument10 pagesNeonatal: Hypoglycemiamarkus_danusantosoNo ratings yet
- Metformin in PCOSDocument5 pagesMetformin in PCOSbalaramNo ratings yet
- Persistent Diarrhea With Severe Malnutrition Type MarasmusDocument47 pagesPersistent Diarrhea With Severe Malnutrition Type MarasmusAkash KhannaNo ratings yet
- Inborn Errors of Metabolism in Infancy - A Guide To DiagnosisDocument11 pagesInborn Errors of Metabolism in Infancy - A Guide To Diagnosismaxime wotolNo ratings yet
- 2018 Article 9455Document21 pages2018 Article 9455ნათია დემეტრაძეNo ratings yet
- Inborn Error of Metabolism and Introduction To Cancer GeneticsDocument9 pagesInborn Error of Metabolism and Introduction To Cancer GeneticsviancaNo ratings yet
- Osama Hamdy, MD, PHD Chief Editor Romesh Khardori, MD, PHD, FACP Etiology Obestity MedscapeDocument4 pagesOsama Hamdy, MD, PHD Chief Editor Romesh Khardori, MD, PHD, FACP Etiology Obestity MedscapeEvi BaeNo ratings yet
- The Pathophysiology of Intrahepatic Cholestasis of PregnancyDocument13 pagesThe Pathophysiology of Intrahepatic Cholestasis of PregnancyEnrique RosasNo ratings yet
- 1097-0142 (19850101) 55 1+ 230 Aid-Cncr2820551305 3.0.co 2-IDocument8 pages1097-0142 (19850101) 55 1+ 230 Aid-Cncr2820551305 3.0.co 2-IOlga ĆalasanNo ratings yet
- (1875855X - Asian Biomedicine) Classical Galactosemia in A Thai Infant - Case Report and Review of The LiteratureDocument6 pages(1875855X - Asian Biomedicine) Classical Galactosemia in A Thai Infant - Case Report and Review of The LiteratureReuben JosephNo ratings yet
- Acute Fatty Liver of Pregnancy A ThoroughDocument8 pagesAcute Fatty Liver of Pregnancy A ThorougheeeeeeNo ratings yet
- Bitton 2016Document3 pagesBitton 2016Bilel DhaouadiNo ratings yet
- Lomer 2008 (Intolerancia A La Lactosa)Document11 pagesLomer 2008 (Intolerancia A La Lactosa)michellevcfNo ratings yet
- Inborn Errors of Metabolism in Infancy: A Guide To DiagnosisDocument11 pagesInborn Errors of Metabolism in Infancy: A Guide To DiagnosisbentoeNo ratings yet
- Type1Diabetesinpregnancy: David R. Mccance,, Claire CaseyDocument15 pagesType1Diabetesinpregnancy: David R. Mccance,, Claire Caseyjose ricardo escalante perezNo ratings yet
- Kuthiroly2021 Article LipoproteinLipaseDeficiencyDocument7 pagesKuthiroly2021 Article LipoproteinLipaseDeficiencybrajendra singhNo ratings yet
- IEM Guide To Diagnosis B BurtonDocument11 pagesIEM Guide To Diagnosis B BurtonPAOLA JACQUELINE VELEZ PINOSNo ratings yet
- Acute Fatty Liver of Pregnancy: Letter To EditorDocument2 pagesAcute Fatty Liver of Pregnancy: Letter To EditorsaryindrianyNo ratings yet
- HELLP and AFLP PDFDocument5 pagesHELLP and AFLP PDFDesyHandayaniNo ratings yet
- Pregnancy-Related Liver Disorders: Seminar Journal of Clinical and Experimental HepatologyDocument12 pagesPregnancy-Related Liver Disorders: Seminar Journal of Clinical and Experimental HepatologysaryindrianyNo ratings yet
- AFLPDocument3 pagesAFLPsaryindrianyNo ratings yet
- Nelson2013 Ajog PDFDocument7 pagesNelson2013 Ajog PDFsaryindrianyNo ratings yet
- Minakami 2014Document9 pagesMinakami 2014saryindrianyNo ratings yet
- Acute Fatty Liver of Pregnancy: Better Understanding of Pathogenesis and Earlier Clinical Recognition Results in Improved Maternal OutcomesDocument8 pagesAcute Fatty Liver of Pregnancy: Better Understanding of Pathogenesis and Earlier Clinical Recognition Results in Improved Maternal OutcomessaryindrianyNo ratings yet
- ACG Clinical Guideline: Liver Disease and Pregnancy: Practice GuidelinesDocument19 pagesACG Clinical Guideline: Liver Disease and Pregnancy: Practice GuidelinessaryindrianyNo ratings yet
- Management Prior Cesarean DeliveryDocument14 pagesManagement Prior Cesarean DeliveryAditya Arya PutraNo ratings yet
- Induction of LaborDocument124 pagesInduction of LaborFarah DinaNo ratings yet
- Jastrow Et Al-2016-Ultrasound in ObstetricsDocument6 pagesJastrow Et Al-2016-Ultrasound in ObstetricssaryindrianyNo ratings yet
- Acog PB184 Vbac 2017 PDFDocument17 pagesAcog PB184 Vbac 2017 PDFdwipaNo ratings yet
- Birth After Previous Caesarean Section (C Obs 38) Re Write July 2015 1Document27 pagesBirth After Previous Caesarean Section (C Obs 38) Re Write July 2015 1saryindrianyNo ratings yet
- Why Obstetric Patients Are Admitted To Intensive Care Unit A Retrospective StudyDocument3 pagesWhy Obstetric Patients Are Admitted To Intensive Care Unit A Retrospective StudysaryindrianyNo ratings yet
- Causes and Management of Surgical Wound Dehiscence: Department of General SurgeryDocument10 pagesCauses and Management of Surgical Wound Dehiscence: Department of General SurgerysaryindrianyNo ratings yet
- VANCOUVER Reference GuideDocument7 pagesVANCOUVER Reference GuideNoval FarlanNo ratings yet
- 3881825Document30 pages3881825saryindrianyNo ratings yet
- Risk Factors For Wound Dehiscence After Laparotomy - Clinical Control TrialDocument9 pagesRisk Factors For Wound Dehiscence After Laparotomy - Clinical Control TrialsaryindrianyNo ratings yet
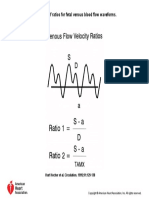
- Calculation of Ratios For Fetal Venous Blood Flow WaveformsDocument1 pageCalculation of Ratios For Fetal Venous Blood Flow WaveformssaryindrianyNo ratings yet
- Partograph 1Document22 pagesPartograph 1SIRBENZNo ratings yet
- Hiperemesis Gravidea PDFDocument7 pagesHiperemesis Gravidea PDFFrank Luis Acosta MendozaNo ratings yet
- Risk Factors For Wound Dehiscence After Laparotomy - Clinical Control TrialDocument9 pagesRisk Factors For Wound Dehiscence After Laparotomy - Clinical Control TrialsaryindrianyNo ratings yet
- Causes and Management of Surgical Wound Dehiscence: Department of General SurgeryDocument10 pagesCauses and Management of Surgical Wound Dehiscence: Department of General SurgerysaryindrianyNo ratings yet
- 1 s2.0 S0889854515001163Document18 pages1 s2.0 S0889854515001163saryindrianyNo ratings yet
- Post Caesarean Surgical Site Infections 2Document6 pagesPost Caesarean Surgical Site Infections 2saryindrianyNo ratings yet
- Renal Vein Doppler Ultrasound of Maternal KidneysDocument5 pagesRenal Vein Doppler Ultrasound of Maternal KidneyssaryindrianyNo ratings yet
- Keamanan Obat Bagi Ibu Hamil Berdasarkan Evidence BasedDocument17 pagesKeamanan Obat Bagi Ibu Hamil Berdasarkan Evidence BasedJasonJejametNo ratings yet
- Diagnosis and Management of Placenta PreviaDocument6 pagesDiagnosis and Management of Placenta PreviaNoveno Semestre FmuaqNo ratings yet
- Technical Series 2 Using PartographDocument5 pagesTechnical Series 2 Using PartographsaryindrianyNo ratings yet
- Guidelines TX of HPNDocument3 pagesGuidelines TX of HPNjheyfteeNo ratings yet
- Jaw Opening Exercises Head and Neck Cancer - Jun21Document2 pagesJaw Opening Exercises Head and Neck Cancer - Jun21FalconNo ratings yet
- ResumeDocument3 pagesResumeAstig Kuging63% (8)
- Product Risk Management Report For Spine ProductsDocument25 pagesProduct Risk Management Report For Spine ProductsAlejandro Landinez100% (1)
- Neem TreeDocument4 pagesNeem Treedorzky22No ratings yet
- C1 Mod 2 - Introduction To Psychoactive Substance UseDocument22 pagesC1 Mod 2 - Introduction To Psychoactive Substance UsePUSAT LATIHAN AADKNo ratings yet
- Problem-Solution Essay FinalDocument8 pagesProblem-Solution Essay FinalNoriko May ManarinNo ratings yet
- ASEAN Guideline On Stability of Drug ProductDocument8 pagesASEAN Guideline On Stability of Drug ProductSanjiv MenonNo ratings yet
- Bios Life Slim UAEDocument4 pagesBios Life Slim UAEJey BautistaNo ratings yet
- 06 Senior Citizen PolicyDocument26 pages06 Senior Citizen PolicyJaimson FrancisNo ratings yet
- Myles Textbook For Midwives, 15th Edition: Journal of Obstetrics and GynaecologyDocument3 pagesMyles Textbook For Midwives, 15th Edition: Journal of Obstetrics and Gynaecologyintan wahyuNo ratings yet
- Writing Nursing Sample Test 2 2014Document4 pagesWriting Nursing Sample Test 2 2014Praveen B S PrasadNo ratings yet
- Budwig Cancer GuideDocument125 pagesBudwig Cancer GuideDavid Gristan100% (1)
- NRSG - Documentation and Quality Management in IvtDocument12 pagesNRSG - Documentation and Quality Management in IvtRamon Carlo AlmiranezNo ratings yet
- Phytochemical Screening and Antimicrobial Assay of Various SeedsDocument9 pagesPhytochemical Screening and Antimicrobial Assay of Various SeedsWendy FXNo ratings yet
- 1Document6 pages1Thái Đàm VănNo ratings yet
- Classification and Patient Selection in Abdominoplasty: Alan Matarasso, MD, FacsDocument8 pagesClassification and Patient Selection in Abdominoplasty: Alan Matarasso, MD, FacsPopa FlorinNo ratings yet
- VOLUX 尖沙咀 chung parr hall 鍾伯豪醫生 PDFDocument8 pagesVOLUX 尖沙咀 chung parr hall 鍾伯豪醫生 PDFAnonymous YT4f4ejNtH100% (1)
- IRBD Scales Abnormal Involuntary Movement ScaleDocument2 pagesIRBD Scales Abnormal Involuntary Movement ScaleRoberto LlanesNo ratings yet
- Herniated Nucleus Pulposus (HNP)Document21 pagesHerniated Nucleus Pulposus (HNP)Kaye100% (1)
- Osma y Barlow 2021 PU en SpainDocument15 pagesOsma y Barlow 2021 PU en Spaingerard sansNo ratings yet
- Article-PDF-sheen Juneja Aman Arora Shushant Garg Surbhi-530Document3 pagesArticle-PDF-sheen Juneja Aman Arora Shushant Garg Surbhi-530JASPREETKAUR0410No ratings yet
- EYE Emergency Manual An Illustrated Guide: Second EditionDocument56 pagesEYE Emergency Manual An Illustrated Guide: Second Editionmanleyj5305No ratings yet
- Data Sheet Data Sheet: Gelafusal GelafusalDocument2 pagesData Sheet Data Sheet: Gelafusal Gelafusalfahri azwarNo ratings yet
- Radiology X-Rayfilm ScreensDocument39 pagesRadiology X-Rayfilm ScreensFourthMolar.comNo ratings yet
- MSDS - Dust SuppressantDocument3 pagesMSDS - Dust SuppressantJc RamírezNo ratings yet
- Awake Craniotomy and Excision of A Diffuse Low Grade Glioma in A Multilingual PatientDocument7 pagesAwake Craniotomy and Excision of A Diffuse Low Grade Glioma in A Multilingual PatientNatalyNo ratings yet
- Medical Terminology SyllabusDocument3 pagesMedical Terminology SyllabusSalman AfrozeNo ratings yet
- 1 MB CVDocument2 pages1 MB CVMichael BonettNo ratings yet
- Tropisetron 26Document6 pagesTropisetron 26Tori SepriwanNo ratings yet