Professional Documents
Culture Documents
Electrochemsitry and Solid State Test Cbse
Uploaded by
prathmfedCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Electrochemsitry and Solid State Test Cbse
Uploaded by
prathmfedCopyright:
Available Formats
TEST ON ELECTROCHEMISTRY AND SOLID STATE (CBSE) TOTAL MARKS: 60
ELECTROCHEMISTRY (30 MARKS)
1. Calculate the potential of hydrogen electrode in contact with a soln whose pH is 6. (2M)
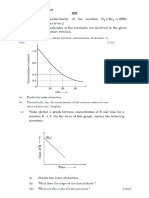
2. How does conductivity of an electrolyte vary with concentration? (3M)
3. Define a fuel cell. Write the cell reactions of fuel cell. (3M)
4. Also state the Kohlrausch law and write the mathematical expression of molar conductivity of the
given solution at infinite dilution. (3M)
5. Estimate the mass of copper metal produced during the passage of 5A current through CuSO4 solution
for 100 minutes. The molar mass of Cu is 63.5g/mol. (3M)
6. Given cell at 298K Cd|Cd ||Ni (2M)| Ni has an emf of 0.20V. Write the cell reactions and ΔG0 for the
2+ +2
cell. What is the molar concentration of Cd2+ ion in this cell? E0cell=0.15V (3M)
7. The conductivity of 0.20M soln of KCl at 298K is 0.0248 S/cm. Calculate the molar conductivity.
Also calculate Λm0 for CaCl2 from the following data. Λ Ca+2=119 S cm2/mol ΛCl-=76.3. (3M)
8. Write the cell notation for the reaction taking place Mg + 2Ag+ (0.1M) Mg2+ (0.001M) + 2Ag.
Calculate Ecell at 298K if E0cell=3.17V. (3M)
+ 2+ 0
9. Calculate Kc for the reaction Cu + 2Ag Cu + 2Ag. E cell=0.58V (3M)
10. Calculate the quantity of electricity that would be required to reduce 12.3gms of nitrobenzene to
aniline, if the current efficiency for the process is 50%. If the potential drops across the cell is 3V, how
much energy will be consumed? (4M)
SOLID STATE (30 MARKS)
11. Calcium crystallises in FCC unit cell with 0.556nm. Calculate the density if it contains 0.2% frenkel
defects. Mol wt of calcium=40 gm/mol (3M)
2+ th 3+
12. In spinel, Mg is present in 1/8 of TV’s in an FCC lattice of oxide ions and Al ions are present in half
of the OV’s. The formula of spinel is____________. (3M)
13. A face centered cubic lattice is made up of single type of atom and one of its corner is left unoccupied
per unit cell. Calculate the packing fraction of such solid. (3M)
14. FeO crystallises in NaCl type of crystal lattice. Some cationic sites are vacant and some contain Fe3+
ions but the structure is electrically neutral. The formula approximates to Fe0.95O1.00. a) What is the
ratio of Fe2+ to Fe3+ ions in solid? b) What percentage of cationic sites are vacant? (3M)
3
15. Density of one form of CaCO3, solid is 2.93gm/cm and it has orthorhombic unit cell with unit cell
parameter: a= 4.6Ao, b= 5.7Ao and c=8Ao. Calculate the number of Ca2+ ions per unit cell. (3M)
16. A compound is made of particles A and B. A forms FCC packing and B occupies all the OVs. If all the
particles along the plane as shown in the figure below are removed, then the simplest formula of the
compound is: - a) A5B7 b) A7B5 c) AB d) AB3.75 (3M)
TEST ON ELECTROCHEMISTRY AND SOLID STATE (CBSE) TOTAL MARKS: 60
17. *In a solid having rock salt structure, if all the atoms touching one body diagonal plane are removed
(except at body centre), then the formula of left unit cell is: - (3M)
a) A3.5B2.5 b) A7B3 c) A5B3 d) A3B5
18. Figure shows unit cell of superconductor. The formula of mixed oxide is YaBabCucOd. Find value of
a+b+c+d. (4M)
19. The percentage packing efficiency of the two dimensional arrangement (2D) of sphere for plane
ABCDEF shown below is: (5M)
a) 90.64% b) 74.05% c) 68.02% d) 78.54%
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (120)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Electronic Fetal MonitoringDocument4 pagesElectronic Fetal MonitoringMauZungNo ratings yet
- How To Defend The Faith Without Raising Your VoiceDocument139 pagesHow To Defend The Faith Without Raising Your VoiceCleber De Souza Cunha100% (2)
- Mpez-6 Installation Manual - Inline 201007Document8 pagesMpez-6 Installation Manual - Inline 201007api-244989438No ratings yet
- DPP-01 Chemical KineticsDocument1 pageDPP-01 Chemical Kineticsprathmfed100% (1)
- Assignment On Coordination CompoundsDocument8 pagesAssignment On Coordination CompoundsprathmfedNo ratings yet
- Chemical Kinetics 2020-2022Document6 pagesChemical Kinetics 2020-2022prathmfedNo ratings yet
- QSN Bank On Aldehydes 2012-2022Document26 pagesQSN Bank On Aldehydes 2012-2022prathmfedNo ratings yet
- QSN Bank On Coordination Compounds 2012-2022Document10 pagesQSN Bank On Coordination Compounds 2012-2022prathmfedNo ratings yet
- Assignment On Solid State, Solutions and KineticsDocument12 pagesAssignment On Solid State, Solutions and KineticsprathmfedNo ratings yet
- Assignment On Nomenclature of Aldehyde-KetonesDocument2 pagesAssignment On Nomenclature of Aldehyde-KetonesprathmfedNo ratings yet
- DPP 19Document1 pageDPP 19prathmfedNo ratings yet
- Assignment On Aldehydes-Ketones-Carboxylic AcidsDocument2 pagesAssignment On Aldehydes-Ketones-Carboxylic AcidsprathmfedNo ratings yet
- Cblechpl 06125Document9 pagesCblechpl 06125prathmfedNo ratings yet
- DPP 18Document1 pageDPP 18prathmfedNo ratings yet
- MCQS'S On Solutions MARKS: 50X4 200M TIME:150MIN: Y Y X X Y Y X XDocument1 pageMCQS'S On Solutions MARKS: 50X4 200M TIME:150MIN: Y Y X X Y Y X XprathmfedNo ratings yet
- DPP 17Document1 pageDPP 17Saiprasad K. MalekarNo ratings yet
- Thermodynamics Assignment-3Document2 pagesThermodynamics Assignment-3prathmfedNo ratings yet
- Thermodynamics Assignment-3Document2 pagesThermodynamics Assignment-3prathmfedNo ratings yet
- Jee Mains 2018Document2 pagesJee Mains 2018prathmfedNo ratings yet
- DPP 02Document1 pageDPP 02prathmfedNo ratings yet
- Jee Advanced Test On EquilibriaDocument2 pagesJee Advanced Test On EquilibriaprathmfedNo ratings yet
- Chemical Kinetics (Order/First Order/Half - Life) Code: 2019-20/3Document1 pageChemical Kinetics (Order/First Order/Half - Life) Code: 2019-20/3prathmfedNo ratings yet
- Yet QSNS On Mole ConceptDocument2 pagesYet QSNS On Mole ConceptprathmfedNo ratings yet
- Qsns On Chemical KineticsDocument1 pageQsns On Chemical KineticsprathmfedNo ratings yet
- Jee Advanced Test On EquilibriaDocument2 pagesJee Advanced Test On EquilibriaprathmfedNo ratings yet
- School Lesson PlanDocument17 pagesSchool Lesson PlanprathmfedNo ratings yet
- Jee Mains 2018Document2 pagesJee Mains 2018prathmfedNo ratings yet
- Lesson 7 EnergyDocument16 pagesLesson 7 EnergyprathmfedNo ratings yet
- School Lesson PlanDocument17 pagesSchool Lesson PlanprathmfedNo ratings yet
- Lesson 8 MirobesDocument11 pagesLesson 8 MirobesprathmfedNo ratings yet
- Classification of PlantsDocument12 pagesClassification of Plantsprathmfed100% (1)
- Lesson 2 WorkDocument15 pagesLesson 2 WorkprathmfedNo ratings yet
- Copy of HW UMTS KPIsDocument18 pagesCopy of HW UMTS KPIsMohamed MoujtabaNo ratings yet
- Hydroprocessing Pilot PlantsDocument4 pagesHydroprocessing Pilot PlantsNattapong PongbootNo ratings yet
- What Is An Engineering Change OrderDocument3 pagesWhat Is An Engineering Change OrderKundan Kumar MishraNo ratings yet
- 18-MCE-49 Lab Session 01Document5 pages18-MCE-49 Lab Session 01Waqar IbrahimNo ratings yet
- Ppr.1 Circ.5 Gesamp Ehs ListDocument93 pagesPpr.1 Circ.5 Gesamp Ehs ListTRANNo ratings yet
- PNFDocument51 pagesPNFMuhamad Hakimi67% (3)
- Fitness Program: Save On Health Club Memberships, Exercise Equipment and More!Document1 pageFitness Program: Save On Health Club Memberships, Exercise Equipment and More!KALAI TIFYNo ratings yet
- 120-202 Lab Manual Spring 2012Document107 pages120-202 Lab Manual Spring 2012evacelon100% (1)
- Ceilcote 222HT Flakeline+ds+engDocument4 pagesCeilcote 222HT Flakeline+ds+englivefreakNo ratings yet
- HOME TECH - HOME TEXTILE REVIEW. Ayman SatopayDocument12 pagesHOME TECH - HOME TEXTILE REVIEW. Ayman SatopayAyman SatopayNo ratings yet
- (Jill E. Thistlethwaite) Values-Based Interprofess (B-Ok - CC)Document192 pages(Jill E. Thistlethwaite) Values-Based Interprofess (B-Ok - CC)Ria Qadariah AriefNo ratings yet
- LTHE Comments On APG's Proposal No. 9090/3181-L&T-Detailed Engineering Services For EPCC-1-AVU Unit, Barauni RefineryDocument9 pagesLTHE Comments On APG's Proposal No. 9090/3181-L&T-Detailed Engineering Services For EPCC-1-AVU Unit, Barauni RefineryajayNo ratings yet
- Week 4 (Theories)Document15 pagesWeek 4 (Theories)Erica Velasco100% (1)
- UNIT 3 Polymer and Fuel ChemistryDocument10 pagesUNIT 3 Polymer and Fuel Chemistryld6225166No ratings yet
- Red Bank Squadron - 01/22/1942Document28 pagesRed Bank Squadron - 01/22/1942CAP History LibraryNo ratings yet
- Julie Trimarco: A Licensed Speech-Language PathologistDocument5 pagesJulie Trimarco: A Licensed Speech-Language PathologistJulie TrimarcoNo ratings yet
- Keith UrbanDocument2 pagesKeith UrbanAsh EnterinaNo ratings yet
- Chemsheets AS 006 (Electron Arrangement)Document27 pagesChemsheets AS 006 (Electron Arrangement)moiz427No ratings yet
- Advances in Agronomy v.84Document333 pagesAdvances in Agronomy v.84luisiunesNo ratings yet
- DoveDocument11 pagesDovekattyperrysherryNo ratings yet
- Tinh Toan Tang AP Cau Thang - CT Qui LongDocument20 pagesTinh Toan Tang AP Cau Thang - CT Qui Longntt_121987No ratings yet
- Harvard Referencing GuideDocument6 pagesHarvard Referencing GuideKhánh Nguyên VõNo ratings yet
- Toolbox Talks - Near Miss ReportingDocument1 pageToolbox Talks - Near Miss ReportinganaNo ratings yet
- NFPA 220 Seminar OutlineDocument2 pagesNFPA 220 Seminar Outlineveron_xiiiNo ratings yet
- The Importance of Early Childhood InterventionDocument11 pagesThe Importance of Early Childhood Interventionsilverlining0814100% (3)
- Prestress 3.0Document10 pagesPrestress 3.0Jonel CorbiNo ratings yet
- Case Analysis: Beth OwensDocument8 pagesCase Analysis: Beth OwensPhillip CookNo ratings yet