Professional Documents
Culture Documents
MCQS'S On Solutions MARKS: 50X4 200M TIME:150MIN: Y Y X X Y Y X X
Uploaded by
prathmfed0 ratings0% found this document useful (0 votes)
27 views1 pagemcq's
Original Title
solution
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this Documentmcq's
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
27 views1 pageMCQS'S On Solutions MARKS: 50X4 200M TIME:150MIN: Y Y X X Y Y X X
Uploaded by
prathmfedmcq's
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
You are on page 1of 1
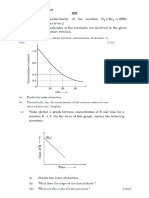
MCQS’S ON SOLUTIONS MARKS: 50X4=200M TIME:150MIN
1. The vapour pressure of a given liquid will
decrease if:
a) surface area of liquid is decreased.
b) The volume of the liquid in the container
is decreased.
c) The volume of vapour phase is increased.
d) The temperature is decreased.
2. Raoult’s law is obeyed by each constituent
of a binary liquid solution when:
a) the forces of attraction between like
molecules are greater than those between
unlike molecules.
b) The forces of attraction between like
molecules are smaller than those between
unlike molecules.
c) The forces of attraction between like
molecules are identical with those between
unlike molecules.
d) The volume occupied by unlike molecules
are different.
3. For an ideal binary liquid solution with
PA0> PB0, which relation between xA and yA is
correct? ( xA and yA are mole fraction of
component A in liquid phase and vapour
phase respectively.)
a) yA > yB b) xA > xB
Y𝐴 X𝐴 Y X𝐴
c) Y𝐵
> X𝐵
d) Y 𝐴 < X𝐵
𝐵
4. An ideal solution is formed by mixing two
volatile liquids A and B. XA and XB are the
mole fractions of A and B respectively in the
solution and YA and YB are the mole
fractions of A and B respectively in the
1
vapour phase. A plot of along y-axis
Y𝐴
1
against X𝐴
along x-axis gives a straight
line. What is the slope of straight line?
A)
You might also like
- Chemistry Class 12 Revision MaterialDocument52 pagesChemistry Class 12 Revision Materialkrish dabhiNo ratings yet
- Class XII - Study Material - ChemistryDocument53 pagesClass XII - Study Material - ChemistryUnwantedNo ratings yet
- Lect.12 Ion Exchange FinalDocument39 pagesLect.12 Ion Exchange FinalDr.D.SELVI100% (5)
- Mass Transfer 1 CLB 20804Document54 pagesMass Transfer 1 CLB 20804muhammad fazmiNo ratings yet
- Solution Appendix-Ii: Vapour Pressure of Pure Liquid and SolutionDocument4 pagesSolution Appendix-Ii: Vapour Pressure of Pure Liquid and SolutionSrijan JaiswalNo ratings yet
- Assignment 2 (7.4.2022) - 24921131Document5 pagesAssignment 2 (7.4.2022) - 24921131Harshwardhan Singh Thakur 7No ratings yet
- 3.4.THERMODYNAMICS OF SOLUTIONS - SOME BASIC CONCEPTS - BusmeonDocument5 pages3.4.THERMODYNAMICS OF SOLUTIONS - SOME BASIC CONCEPTS - BusmeonAJ Kent Ray BusmeonNo ratings yet
- CBSE Class 12 Chem Notes Question Bank Solutions PDFDocument16 pagesCBSE Class 12 Chem Notes Question Bank Solutions PDFMonika AdhikariNo ratings yet
- SolutionsDocument5 pagesSolutionsPranav ShinojNo ratings yet
- Solutions - Practice Sheet - VIJETA SERIES CLASS-12THDocument6 pagesSolutions - Practice Sheet - VIJETA SERIES CLASS-12THpragy SinhaNo ratings yet
- Soal Titrasi Asam Basa1 (Annisa Rahmah 17176001)Document3 pagesSoal Titrasi Asam Basa1 (Annisa Rahmah 17176001)annisa rahmahNo ratings yet
- 12 Chemistry English 2020 21Document360 pages12 Chemistry English 2020 21Aanchal SaranNo ratings yet
- Solutions CLASS 12 PDF QUESTIONSDocument35 pagesSolutions CLASS 12 PDF QUESTIONSJampa SaicharanNo ratings yet
- Properties of MixtureDocument26 pagesProperties of MixtureDuy Anh ĐàoNo ratings yet
- Differential EquationsDocument25 pagesDifferential EquationsNilo Daniel MielesNo ratings yet
- Chemistry (PT-2) : Topic: SolutionDocument2 pagesChemistry (PT-2) : Topic: SolutionchitranshNo ratings yet
- Solutions NCERTDocument16 pagesSolutions NCERTPrecisive OneNo ratings yet
- Topic:-: SolutionsDocument3 pagesTopic:-: SolutionsGnaneshwarNo ratings yet
- Learning Materials of Chemistry For Board ExamDocument80 pagesLearning Materials of Chemistry For Board Examnwork0274No ratings yet
- Solution Tsd3Document3 pagesSolution Tsd3Mahendra RajpootNo ratings yet
- Solutions+ +Top+Practice+Problems++ (19!04!2021)Document57 pagesSolutions+ +Top+Practice+Problems++ (19!04!2021)Ritheesh NagarajanNo ratings yet
- SOLUTIONSDocument15 pagesSOLUTIONSbijjaljosephNo ratings yet
- Solution Learning PointsDocument19 pagesSolution Learning Pointstiwari_anunay1689No ratings yet
- 12 Chemistry Keypoints Revision Questions Chapter 2Document15 pages12 Chemistry Keypoints Revision Questions Chapter 2aesthetic rushNo ratings yet
- Test Bank SolutionsDocument47 pagesTest Bank SolutionsMohammed AhmedNo ratings yet
- PDF PDFDocument3 pagesPDF PDFAshwin NairNo ratings yet
- Gcesoln 7Document7 pagesGcesoln 7api-3734333No ratings yet
- Distillation ChEN DLSUDocument2 pagesDistillation ChEN DLSUJelain HumarangNo ratings yet
- MostlikelyquestionsDocument19 pagesMostlikelyquestionsendtimes066xNo ratings yet
- Study Material Chemistry 2022-23Document166 pagesStudy Material Chemistry 2022-23Akash Kumar UpadhyayNo ratings yet
- (Viii) Formality (F) - : Mass of The Ionic Solute in Gram Per Litre Formula Mass of The SolutionDocument1 page(Viii) Formality (F) - : Mass of The Ionic Solute in Gram Per Litre Formula Mass of The SolutionAakash MalviyaNo ratings yet
- CH 2 1Document15 pagesCH 2 1Mohammed AmmaarNo ratings yet
- Wa0006.Document3 pagesWa0006.YOGA SAI GUHANNo ratings yet
- 14A-Chapter 14, Secs 14.1 - 14.7 BlackDocument18 pages14A-Chapter 14, Secs 14.1 - 14.7 BlackArunNo ratings yet
- lbc2 PDFDocument9 pageslbc2 PDFKwin CaragNo ratings yet
- Ion Exchange - Treatment-2Document86 pagesIon Exchange - Treatment-2Shabbir OsmaniNo ratings yet
- Xicbse-Chemistry Asst 2Document3 pagesXicbse-Chemistry Asst 2tanishkakannan3253No ratings yet
- Reverse Osmosis.: EnglishDocument27 pagesReverse Osmosis.: EnglishGøbindNo ratings yet
- 6.4 Solutions Edited 2020Document62 pages6.4 Solutions Edited 2020Anisha Syazwana Binti RoslyNo ratings yet
- Unit 2 Solutions: Points To RememberDocument15 pagesUnit 2 Solutions: Points To RememberHarpreet kaurNo ratings yet
- Target 1 - Level 2 - Chapter 2 PDFDocument26 pagesTarget 1 - Level 2 - Chapter 2 PDFRohan ArekatlaNo ratings yet
- 6645646Document2 pages6645646honeylet tayactacNo ratings yet
- 03ADifusion Mass TransferDocument22 pages03ADifusion Mass TransferIhya UlumudinNo ratings yet
- Exemplar SolutionsDocument20 pagesExemplar Solutionsdrishtidalakoti24001No ratings yet
- Units of SolutionsDocument17 pagesUnits of SolutionsamoNo ratings yet
- CH1 Soution HHW Worksheet1Document6 pagesCH1 Soution HHW Worksheet1Aaditya SharmaNo ratings yet
- ChemDocument9 pagesChemSagar SharmaNo ratings yet
- MRT MDocument8 pagesMRT MSrijan JaiswalNo ratings yet
- Alizar, PH.D: Fakultas Matematika Dan Ilmu Pengetahuan Alam Universitas Negeri PadangDocument27 pagesAlizar, PH.D: Fakultas Matematika Dan Ilmu Pengetahuan Alam Universitas Negeri PadangNathanNo ratings yet
- Concentration Units (Theory For Lesson N 3)Document7 pagesConcentration Units (Theory For Lesson N 3)Shahebaz KhanNo ratings yet
- Warmup 20222Document2 pagesWarmup 20222rania khasawnehNo ratings yet
- 1.a. Fundamentals of Mass TransferDocument17 pages1.a. Fundamentals of Mass TransferThaer MarwanNo ratings yet
- Solution AssignmentDocument4 pagesSolution AssignmentAnkitha shajiNo ratings yet
- Sheet - 01 (Solution) Liquid Theory NJ - 247Document31 pagesSheet - 01 (Solution) Liquid Theory NJ - 247Bhola SolankiNo ratings yet
- Chemistry: SolutionDocument68 pagesChemistry: SolutionSatyajit RoutNo ratings yet
- Mcq-Sol, Elctro, Chemical Kine PDFDocument24 pagesMcq-Sol, Elctro, Chemical Kine PDFTaranjot SinghNo ratings yet
- NEET UG Chemistry Solutions PDFDocument31 pagesNEET UG Chemistry Solutions PDFAlexa Siddhi0% (1)
- Solution NotesDocument110 pagesSolution NotesMomosNo ratings yet
- Holiday Homework Class Xii-ADocument42 pagesHoliday Homework Class Xii-Ageetanjaliawasthi375No ratings yet
- DPP-01 Chemical KineticsDocument1 pageDPP-01 Chemical Kineticsprathmfed100% (1)
- Assignment On Coordination CompoundsDocument8 pagesAssignment On Coordination CompoundsprathmfedNo ratings yet
- Chemical Kinetics 2020-2022Document6 pagesChemical Kinetics 2020-2022prathmfedNo ratings yet
- QSN Bank On Aldehydes 2012-2022Document26 pagesQSN Bank On Aldehydes 2012-2022prathmfedNo ratings yet
- QSN Bank On Coordination Compounds 2012-2022Document10 pagesQSN Bank On Coordination Compounds 2012-2022prathmfedNo ratings yet
- Assignment On Solid State, Solutions and KineticsDocument12 pagesAssignment On Solid State, Solutions and KineticsprathmfedNo ratings yet
- Assignment On Nomenclature of Aldehyde-KetonesDocument2 pagesAssignment On Nomenclature of Aldehyde-KetonesprathmfedNo ratings yet
- DPP 19Document1 pageDPP 19prathmfedNo ratings yet
- Assignment On Aldehydes-Ketones-Carboxylic AcidsDocument2 pagesAssignment On Aldehydes-Ketones-Carboxylic AcidsprathmfedNo ratings yet
- Cblechpl 06125Document9 pagesCblechpl 06125prathmfedNo ratings yet
- DPP 18Document1 pageDPP 18prathmfedNo ratings yet
- Electrochemsitry and Solid State Test CbseDocument2 pagesElectrochemsitry and Solid State Test CbseprathmfedNo ratings yet
- DPP 17Document1 pageDPP 17Saiprasad K. MalekarNo ratings yet
- Thermodynamics Assignment-3Document2 pagesThermodynamics Assignment-3prathmfedNo ratings yet
- Thermodynamics Assignment-3Document2 pagesThermodynamics Assignment-3prathmfedNo ratings yet
- Jee Mains 2018Document2 pagesJee Mains 2018prathmfedNo ratings yet
- DPP 02Document1 pageDPP 02prathmfedNo ratings yet
- Jee Advanced Test On EquilibriaDocument2 pagesJee Advanced Test On EquilibriaprathmfedNo ratings yet
- Chemical Kinetics (Order/First Order/Half - Life) Code: 2019-20/3Document1 pageChemical Kinetics (Order/First Order/Half - Life) Code: 2019-20/3prathmfedNo ratings yet
- Yet QSNS On Mole ConceptDocument2 pagesYet QSNS On Mole ConceptprathmfedNo ratings yet
- Qsns On Chemical KineticsDocument1 pageQsns On Chemical KineticsprathmfedNo ratings yet
- Jee Advanced Test On EquilibriaDocument2 pagesJee Advanced Test On EquilibriaprathmfedNo ratings yet
- School Lesson PlanDocument17 pagesSchool Lesson PlanprathmfedNo ratings yet
- Jee Mains 2018Document2 pagesJee Mains 2018prathmfedNo ratings yet
- Lesson 7 EnergyDocument16 pagesLesson 7 EnergyprathmfedNo ratings yet
- School Lesson PlanDocument17 pagesSchool Lesson PlanprathmfedNo ratings yet
- Lesson 8 MirobesDocument11 pagesLesson 8 MirobesprathmfedNo ratings yet
- Classification of PlantsDocument12 pagesClassification of Plantsprathmfed100% (1)
- Lesson 2 WorkDocument15 pagesLesson 2 WorkprathmfedNo ratings yet