Professional Documents
Culture Documents
App Thermoynamics 2015 Unit TST 1
Uploaded by
Gourab ChakrabortyOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
App Thermoynamics 2015 Unit TST 1
Uploaded by
Gourab ChakrabortyCopyright:
Available Formats
APPLIED THERMODYNAMICS (ME 301)
TIME: 1HOUR 30MINUTES UNIT TEST 1 FULL MARKS :30
Steam table and mollier chart to be supplied to the exam centre.
Your answer should be brief. please avoid Unnecessary & irrelevant discussions
Give diagrams whenever necessary
Group- A
(Answer any six) 2.5×6=15
1. Prove that energy is a property of the system.
2. What is “quasi-static process”?
3. What do you mean by path function and point function. Give examples of both.
4. What is state’s postulate?
5. Define : open system , closed system and isolated system.
6. State zeroth law.
7. DH=dE +d(PV) ,then in an isothermal reversible process dE=0 and d(PV) =Work done= -nRT2.303log (final
volume/initial volume) therefore dH is not equal to 0, then why it is said that change of enthalpy in an
isothermal reversible process is 0?
8. We find the cop of refrigerator and not efficiency. Why?
9. An automobile engine produces 136 hp on the on the output shaft with a thermal efficiency of 30%.the
fuel it burns gives35000 kj/kg energy release. Find out the rate of fuel consumption.
10. What do you understand by the mean temperature of heat addition in vapour power cycle?
Group –B
(Long answer type questions; answer any 3) 5×3=15
11. Prove that if kelvin plank system is violated clausius statement will also be violated.
12. State and prove carnot theorem.
13. Find out : steady flow energy equation with suitable noations.
14. .A 25 kg cast iron wood burning stove, contains 5 kg of soft pine wood and 1kg of air. All the masses are at
room temperature 200 C and pressure 101 kPa. The wood now burns and heats all the mass uniformly,
releasing 1500 W. neglect any air flow and changes in mass of wood and heat losses. Find the rate of
change of temperature (dT/dt) and estimate the time it will take to temperature 750 C.
15. .i> Isentropic process doesn't always imply that it's reversible adiabatic process.- do you support the
statement. Please mention.
ii> “PMM 2 is possible”.- do you support the statement. Justify your answer.
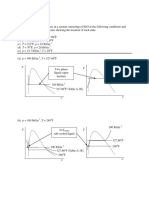
16. 16. Steam at 20 bar, 360°C is expanded in a steam turbine to 0.08 bar. It then enters a condenser, where
it is condensed to saturated liquid water. The pump feeds back the water into the boiler.
(i) Assuming ideal processes, find per kg of steam the net work and the cycle efficiency
(ii) If the turbine and the pump have each 80% efficiency, find the percentage reduction in the net work
and cycle efficiency.
17. 17.An engine working on the Otto cycle is supplied with air at 0.1 MPa ,350 C. The compression ratio is 8.
Heat supplied is 2100 KJ/Kg. Calculate the maximum pressure and temperature of the cycle, the cycle
efficiency and the mean effective pressure. (For air, cp= 1.005, cv= 0.718, and R= 0.287 KJ/Kg.
You might also like
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (121)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (400)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Acid Base BalanceDocument22 pagesAcid Base BalanceSattu SinghNo ratings yet
- Compre Special Project (PPE &IPE)Document240 pagesCompre Special Project (PPE &IPE)Allen Espeleta83% (47)
- Disassociation Constant Estimation Using Acetic Acid and Sodium Hydroxide TitrationDocument19 pagesDisassociation Constant Estimation Using Acetic Acid and Sodium Hydroxide Titrationwani280475% (4)
- Enthalpy-Entropy Diagram For Steam Med 50 MPaDocument1 pageEnthalpy-Entropy Diagram For Steam Med 50 MPaA Christina Hansson100% (2)
- JHAMA JHAM Thermodynamics by NEGI10 (NEGI Sir)Document3 pagesJHAMA JHAM Thermodynamics by NEGI10 (NEGI Sir)VenkataramanaNo ratings yet
- Approval Form - IT - VT - FTDocument5 pagesApproval Form - IT - VT - FTGourab ChakrabortyNo ratings yet
- gv1 PDFDocument1 pagegv1 PDFGourab ChakrabortyNo ratings yet
- Materials Science: Course Motivation and HighlightsDocument4 pagesMaterials Science: Course Motivation and Highlightskh_gallardoNo ratings yet
- VT1Document1 pageVT1Gourab ChakrabortyNo ratings yet
- DRDocument1 pageDRGourab ChakrabortyNo ratings yet
- Project Proposals Proposed by Sayon DeyDocument3 pagesProject Proposals Proposed by Sayon DeyGourab ChakrabortyNo ratings yet
- DRDocument1 pageDRGourab ChakrabortyNo ratings yet
- Title PageDocument1 pageTitle PageGourab ChakrabortyNo ratings yet
- AquaponicsDocument3 pagesAquaponicsGourab Chakraborty0% (1)
- NDT 11.07.2016Document4 pagesNDT 11.07.2016Gourab ChakrabortyNo ratings yet
- Repot On NDTDocument2 pagesRepot On NDTGourab ChakrabortyNo ratings yet
- Additional Numerical BufferDocument2 pagesAdditional Numerical BufferPrahlad DasNo ratings yet
- Lab 29Document2 pagesLab 29KeenanNo ratings yet
- Gcse Chemistry: UNIT 2.4: FactfileDocument7 pagesGcse Chemistry: UNIT 2.4: FactfileClaresta TjandraNo ratings yet
- Thermodynamics All DerivationsDocument8 pagesThermodynamics All Derivationsanuragrabha99No ratings yet
- BDVDocument3 pagesBDVJason ThomasNo ratings yet
- Enthalpy of Protonation of GlycineDocument6 pagesEnthalpy of Protonation of GlycineNur Syazwana SharimNo ratings yet
- Psychrometric Chart Normal Temperature SI Units Sea Level: Barometric Pressure: 101.325 KpaDocument1 pagePsychrometric Chart Normal Temperature SI Units Sea Level: Barometric Pressure: 101.325 Kpaaya maapNo ratings yet
- Experiment 4 Preparation of Isoamyl AcetateDocument8 pagesExperiment 4 Preparation of Isoamyl Acetatelshan SahaNo ratings yet
- Heat Conduction Equation: BMCG 2123 Faculty of Manufacturing Engineering, Utem Taufik Week 2Document45 pagesHeat Conduction Equation: BMCG 2123 Faculty of Manufacturing Engineering, Utem Taufik Week 2elly_lim_3No ratings yet
- DGT Chemical Thermodynamics PDFDocument20 pagesDGT Chemical Thermodynamics PDFAYUSH SHUKLANo ratings yet
- Lab Manual Exp 1-Mass TransferDocument3 pagesLab Manual Exp 1-Mass TransferChong EkNo ratings yet
- Thermal Radiation: Abolencia, Edin Hayel T., Sanchez, Krizia, Soriano, AllanDocument3 pagesThermal Radiation: Abolencia, Edin Hayel T., Sanchez, Krizia, Soriano, AllanEdin AbolenciaNo ratings yet
- HT MCQ KKDocument76 pagesHT MCQ KKgaur1234No ratings yet
- 6.1 Dynamic Equilibrium 1718Document13 pages6.1 Dynamic Equilibrium 1718Ainaa NajwaaNo ratings yet
- Ali 5Document7 pagesAli 5Losi GrahamNo ratings yet
- β= T T T T E T Q Q: Tutorial Sheet 5 (Document6 pagesβ= T T T T E T Q Q: Tutorial Sheet 5 (deshrajNo ratings yet
- DLP - Class1 - Grp.1 - Day 4 (Heat & Temperature)Document4 pagesDLP - Class1 - Grp.1 - Day 4 (Heat & Temperature)Valdeleon Taguiam CatherineNo ratings yet
- Ch03 ThermoDocument34 pagesCh03 ThermoPa Loma B. SantosNo ratings yet
- Handout 9 ThermodynamicsDocument10 pagesHandout 9 ThermodynamicsMary Grace AcostaNo ratings yet
- FTPDocument8 pagesFTPMartin FuenzalidaNo ratings yet
- Chem 82 Entropy Problem SetDocument1 pageChem 82 Entropy Problem SetJoeMarieValcarcelNo ratings yet
- Thermodynamic Property MethodsDocument23 pagesThermodynamic Property MethodsfarhaNo ratings yet
- Physical ChemistryDocument8 pagesPhysical ChemistryFroileth PulidoNo ratings yet
- ME 231 Montazami Whharris 9-10-18 SOLUTIONDocument4 pagesME 231 Montazami Whharris 9-10-18 SOLUTIONEduardo Perez UriegasNo ratings yet
- T.D, 2 Marks & Notes For Units 1,2,3Document54 pagesT.D, 2 Marks & Notes For Units 1,2,3ABHIROOP KNo ratings yet