Professional Documents
Culture Documents
USER SCOPED TEMP DATA Orca-Image - 1632063042
USER SCOPED TEMP DATA Orca-Image - 1632063042
Uploaded by
ann felaireOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
USER SCOPED TEMP DATA Orca-Image - 1632063042
USER SCOPED TEMP DATA Orca-Image - 1632063042
Uploaded by
ann felaireCopyright:
Available Formats
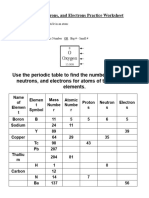
Metropol ita n Institutes of A rts and Scienc es
Proto11 , Neutro11 , and Electron Practice \ ork l1eet
Calculal1ng the nun1ber of each particle 1n an aton1:
=
# Proton, A ton11c Nun1hcr
=
# ElcLlron., Proton
# Neutron = 1\ ton11c 1a\)t - A ton1ic Nun1ber OR Big '# - S1nall #
8
0
Oxygen
t: e the penod1c t ahle to tindlhe nun1bers of proton .neutron • and eleLLron' lor aton1 of the folio\\ 1ng
element.
I
I
Elen1ent ta s Ato 1nic
Nan1e of Elen1ent Proton., Neulrt ll' Electron
S)mbol Number >-1un1ber
Boron B II 5 5 6 5
Sodiun1 24 II
y 89 39
Copper
-
29 35 -
Tc 98 .tJ
Ph 207
Thalliun1 204 81
H 0
Carbon 12
N 7 ,_
- Calciutn
Ba
-
56
Si 14
-
f\rgon 18
1g 12 12
prepared by Ms. Mary Ann S. Felaire
You might also like
- Annotated-Atomic Structure Bohr Models-1Document2 pagesAnnotated-Atomic Structure Bohr Models-1Ivania Joselina Lobo MontoyaNo ratings yet
- Protons, Neutrons, and Electrons Practice Worksheet For 8th GradeDocument2 pagesProtons, Neutrons, and Electrons Practice Worksheet For 8th GradeDrama Music67% (3)
- Product Specification: Enzyme ActivitiesDocument2 pagesProduct Specification: Enzyme ActivitiesrolandoNo ratings yet
- Subatomic Particles WsDocument1 pageSubatomic Particles WsYhena ChanNo ratings yet
- Protons, Neutrons, and Electrons Practice WorksheetDocument1 pageProtons, Neutrons, and Electrons Practice WorksheetRosa SaritaNo ratings yet
- GurrentFlow DiagramDocument8 pagesGurrentFlow DiagramurquattroNo ratings yet
- Worksheet: Atoms, Isotopes, and Ions AtomsDocument2 pagesWorksheet: Atoms, Isotopes, and Ions AtomsLeo Torres GarcíaNo ratings yet
- Group Activity1Document1 pageGroup Activity1ansalerochNo ratings yet
- Kim Lighting Landscape Lighting Catalog 1988Document28 pagesKim Lighting Landscape Lighting Catalog 1988Alan MastersNo ratings yet
- Subatomic Particles - FILLDocument2 pagesSubatomic Particles - FILLALMERA SHELLA CABOGONo ratings yet
- Atomic StructureDocument11 pagesAtomic StructureHamza OmerNo ratings yet
- Jadwal Siswa Sem 1 TP 2022 2023Document36 pagesJadwal Siswa Sem 1 TP 2022 2023Alshafira PutriNo ratings yet
- Unit 2: Atomic Structure & Periodic Table: El-WakilDocument17 pagesUnit 2: Atomic Structure & Periodic Table: El-Wakilmaryamhaitham805No ratings yet
- 1-4 Atomic Structure and Periodic TableDocument13 pages1-4 Atomic Structure and Periodic Table227easonNo ratings yet
- Ion Worksheet KEYDocument1 pageIon Worksheet KEYAna Marie Corales TabunarNo ratings yet
- Protons, Neutrons, and Electrons Practice WorksheetDocument1 pageProtons, Neutrons, and Electrons Practice Worksheetcrisanto cabatbatNo ratings yet
- Kami Export - Youssef Shalaby - Basic Atomic Structure WorksheetDocument1 pageKami Export - Youssef Shalaby - Basic Atomic Structure Worksheetzakisteam128No ratings yet
- Basic Atomic Structure Worksheet ANSWERSDocument2 pagesBasic Atomic Structure Worksheet ANSWERSMiss RonaNo ratings yet
- Chem F4 Teaching HiddenDocument5 pagesChem F4 Teaching HiddenNurul alya Qistina sulaimanNo ratings yet
- Atomic MassDocument1 pageAtomic MassDeepti JainNo ratings yet
- Doping of PolymerDocument16 pagesDoping of Polymergitanjali kashniNo ratings yet
- 1 - Atomic Mass and Atomic Number WorksheetDocument1 page1 - Atomic Mass and Atomic Number WorksheetprevendidosamanthaNo ratings yet
- NucleusDocument2 pagesNucleusSandhyaNo ratings yet
- Periodic Properties 1Document39 pagesPeriodic Properties 1Cat123No ratings yet
- MTC - SSDocument2 pagesMTC - SSSWARNALI DEYNo ratings yet
- Reduction of Graphite PDFDocument5 pagesReduction of Graphite PDFĐổi ThayNo ratings yet
- 2024 - Chapter3 - Atomic StructureDocument15 pages2024 - Chapter3 - Atomic Structurekaylamok3No ratings yet
- Pop 1966 01 PDFDocument116 pagesPop 1966 01 PDFAriosto MartireNo ratings yet
- Elements and The Periodic Table WorksheetDocument4 pagesElements and The Periodic Table WorksheetVictoria StewartsonNo ratings yet
- Tinywow - Chemistry Project - F-Block - PDFDocument27 pagesTinywow - Chemistry Project - F-Block - PDFTHEONLYNABILNo ratings yet
- Electrochemistry Online Class NotesDocument3 pagesElectrochemistry Online Class Notesanas.asif2008No ratings yet
- CH 4Document6 pagesCH 4SujalNo ratings yet
- Protons, Neutrons, and Electrons Practice WorksheetDocument1 pageProtons, Neutrons, and Electrons Practice WorksheetMelerose Dela SernaNo ratings yet
- Basic Atomic Structure Worksheet ANSWERSDocument2 pagesBasic Atomic Structure Worksheet ANSWERSlex marantalNo ratings yet
- Kim Lighting Landscape Lighting Catalog 1991Document28 pagesKim Lighting Landscape Lighting Catalog 1991Alan MastersNo ratings yet
- Paganini Caprice 24 For GuitarDocument7 pagesPaganini Caprice 24 For Guitarjuan carlos Güemes cándida dávalosNo ratings yet
- Lot 1413 & 5988, Pls-2Document1 pageLot 1413 & 5988, Pls-2Reynaldo Delos ReyesNo ratings yet
- Home Activity (Blank Periodic Table) Pe #17Document2 pagesHome Activity (Blank Periodic Table) Pe #17Karylle AguilaNo ratings yet
- NB2039 Kitab Al Awamilun Nahwi Atau Kitab Al Awamil Al Maah Fin Nahwi - 001Document36 pagesNB2039 Kitab Al Awamilun Nahwi Atau Kitab Al Awamil Al Maah Fin Nahwi - 001mustain ahmadNo ratings yet
- Nuclear PhysicsDocument20 pagesNuclear Physicssuneel SaiNo ratings yet
- PG 1Document1 pagePG 1Khoo Rui JieNo ratings yet
- Kim Lighting Landscape Lighting Catalog 1982Document28 pagesKim Lighting Landscape Lighting Catalog 1982Alan MastersNo ratings yet
- Chitty Chitty Bang Bang: ViolaDocument4 pagesChitty Chitty Bang Bang: ViolaMarco ScicliNo ratings yet
- Technology As An Enabler For Marginal Field DevelopmentDocument23 pagesTechnology As An Enabler For Marginal Field DevelopmentSang Duong VanNo ratings yet
- Sun Mar 17 2019 - 8:00 PM: Apple Jean BarcoDocument2 pagesSun Mar 17 2019 - 8:00 PM: Apple Jean BarcoBernLowelNo ratings yet
- Reductive Extraction of Lanthanide and Actinide Elements From Molten LiF BeF2 Salt Into Liquid BismuthDocument10 pagesReductive Extraction of Lanthanide and Actinide Elements From Molten LiF BeF2 Salt Into Liquid Bismuthga6ba5No ratings yet
- ادوت الحفرDocument1 pageادوت الحفرElatif 2007No ratings yet
- Coding DecodingDocument4 pagesCoding DecodingGayathriRajiNo ratings yet
- KCSE Form 2 NotesDocument139 pagesKCSE Form 2 NotesN KatanaNo ratings yet
- MESLDocument4 pagesMESLJim CruzNo ratings yet
- Lesson 1-2Document11 pagesLesson 1-2joelikestwich7070No ratings yet
- Figures 2 32 2-33a B: Repulsion-Type Motors I Lustra Ions-Chapter 2Document1 pageFigures 2 32 2-33a B: Repulsion-Type Motors I Lustra Ions-Chapter 2Jayson Jonson AraojoNo ratings yet
- Atomic: StructureDocument1 pageAtomic: StructureJovariya RaziqNo ratings yet
- Test Project Inc 2022Document6 pagesTest Project Inc 2022yoanaNo ratings yet
- Omega Lighting EF7251-7252-7253-7262 HID MV & MH Cylinder Spec Sheet 11-80Document2 pagesOmega Lighting EF7251-7252-7253-7262 HID MV & MH Cylinder Spec Sheet 11-80Alan MastersNo ratings yet
- The Periodic Table of ElementsDocument23 pagesThe Periodic Table of ElementsKimberly JoyceNo ratings yet
- SDFSFDocument3 pagesSDFSFAmyNo ratings yet
- Img 20230315 0002Document1 pageImg 20230315 0002Jeremiah PrintingNo ratings yet
- XXIVth International Congress of Pure and Applied Chemistry: Plenary and Main Section Lectures Presented at Hamburg, Federal Republic of Germany, 2–8 September 1973From EverandXXIVth International Congress of Pure and Applied Chemistry: Plenary and Main Section Lectures Presented at Hamburg, Federal Republic of Germany, 2–8 September 1973No ratings yet
- Development and Characterization of Nanoemulsion Containing Almond Oil, Biodegradable Polymer and Propranolol As Potential Treatment in HemangiomaDocument11 pagesDevelopment and Characterization of Nanoemulsion Containing Almond Oil, Biodegradable Polymer and Propranolol As Potential Treatment in HemangiomaFabiana OlenaNo ratings yet
- ACI 350R-89 Environmental Engineering Concrete StructuresDocument26 pagesACI 350R-89 Environmental Engineering Concrete StructuresvasanthNo ratings yet
- Mineral: Amethyst QuartzDocument1 pageMineral: Amethyst QuartzTotztutz Togodunz TonztunzNo ratings yet
- Non-Ferrous Metal: Recycling and Pollution ControlDocument3 pagesNon-Ferrous Metal: Recycling and Pollution ControlCarlos BustamanteNo ratings yet
- Spheronizer and Marumerisiers and Other Special Is Ed Granulation andDocument56 pagesSpheronizer and Marumerisiers and Other Special Is Ed Granulation andNitu JhaNo ratings yet
- SND LMV 331 IOM RevaDocument78 pagesSND LMV 331 IOM RevaCarloNo ratings yet
- 2011-Lecture 3-MSI Types and HysteresisDocument14 pages2011-Lecture 3-MSI Types and Hysteresisheartwin1No ratings yet
- FoundryDocument9 pagesFoundryMuhammad Tauseef ZafarNo ratings yet
- Composition of Gipsum PDFDocument4 pagesComposition of Gipsum PDFdwiNo ratings yet
- Chapter 25-Canal Lining: NotesDocument13 pagesChapter 25-Canal Lining: NotesAnonymous oVmxT9KzrbNo ratings yet
- Pharmacy Critical CareDocument136 pagesPharmacy Critical CareTohShengPoo100% (1)
- Ak SteelDocument27 pagesAk SteelbpchimeraNo ratings yet
- Glycerin Base Engine Coolant For Automobile and Light-Duty ServiceDocument5 pagesGlycerin Base Engine Coolant For Automobile and Light-Duty ServiceInsumos GygNo ratings yet
- CHM 8230-1Document41 pagesCHM 8230-1Abba YakubuNo ratings yet
- Cpcs Antidote Chart August 2022Document7 pagesCpcs Antidote Chart August 2022Dominic MugambiNo ratings yet
- Standards Publication Technical Corrigendum: Doc No: QP-STD - S - 036Document24 pagesStandards Publication Technical Corrigendum: Doc No: QP-STD - S - 036samynathan_bvsNo ratings yet
- 0653 w16 QP 51Document12 pages0653 w16 QP 51yuke kristinaNo ratings yet
- SIHI Refinery E09Document4 pagesSIHI Refinery E09pramodtryNo ratings yet
- YeastGuide PosterDocument1 pageYeastGuide PosterOctavio LopezNo ratings yet
- Phases of Matter - Video QuestionsDocument1 pagePhases of Matter - Video Questionsapi-329058682No ratings yet
- Experiment 3:: Determination of Mixed AlkaliDocument24 pagesExperiment 3:: Determination of Mixed AlkaliRaphael E. MiguelNo ratings yet
- Lesson1DIGESTIVESYSTEM DLPDocument11 pagesLesson1DIGESTIVESYSTEM DLPDen Angelica DungoNo ratings yet
- E02 - GpsaDocument6 pagesE02 - GpsaJorge Luis Guerra FlorezNo ratings yet
- Ca-Dc21e Im 96M11555 GB WW 1021-1Document2 pagesCa-Dc21e Im 96M11555 GB WW 1021-1tomy_ueziNo ratings yet
- Ammonia and Urea CycleDocument17 pagesAmmonia and Urea CycleAboubakar Moalim Mahad moh'dNo ratings yet
- Finallllllllllllllllllllllllll Nani 1Document73 pagesFinallllllllllllllllllllllllll Nani 1Angel TagaanNo ratings yet
- Safety Data Sheet: Ultramid B3Eg6 Ungefaerbt NTDocument8 pagesSafety Data Sheet: Ultramid B3Eg6 Ungefaerbt NTSam MalikNo ratings yet
- Type of Construction JointsDocument11 pagesType of Construction JointsHossam KamalNo ratings yet
- PFE Produced Water Treatment TechnologiesDocument91 pagesPFE Produced Water Treatment TechnologiesHassen Gannouni100% (1)