Professional Documents
Culture Documents
Reacciones de Arsenio y Demás
Uploaded by
Fernandita Carla Coro0 ratings0% found this document useful (0 votes)
12 views1 pageThis document describes chemical reactions involving zinc, cobalt, nickel, and manganese. It provides equations for reactions where these metals form hydroxides, sulfides, nitrates, and other compounds when reacted with ammonium hydroxide, ammonium sulfide, hydrochloric acid, sodium hydroxide, and hydrogen peroxide in aqueous solution. Precipitates are shown for solid products.
Original Description:
Original Title
Reacciones de arsenio y demás.docx
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThis document describes chemical reactions involving zinc, cobalt, nickel, and manganese. It provides equations for reactions where these metals form hydroxides, sulfides, nitrates, and other compounds when reacted with ammonium hydroxide, ammonium sulfide, hydrochloric acid, sodium hydroxide, and hydrogen peroxide in aqueous solution. Precipitates are shown for solid products.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
12 views1 pageReacciones de Arsenio y Demás
Uploaded by
Fernandita Carla CoroThis document describes chemical reactions involving zinc, cobalt, nickel, and manganese. It provides equations for reactions where these metals form hydroxides, sulfides, nitrates, and other compounds when reacted with ammonium hydroxide, ammonium sulfide, hydrochloric acid, sodium hydroxide, and hydrogen peroxide in aqueous solution. Precipitates are shown for solid products.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
You are on page 1of 1
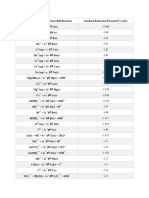
REACCIONES QUÍMICAS:
Zinc (Zn):
Zn+2 (ac) + 2NH4OH (ac)) → Zn(OH)2 (ac) + 2NH4+(ac)
Zn(OH)2 (ac) + (NH4)2S(ac) → ↓ZnS(s) + 2NH4OH(ac)
∆ Zn+2
ZnS(s) + 2HCl(ac) → -
(ac) + 2Cl (ac) + ↑H2S(g)
Zn+2 (ac) + 2NH4Cl(ac) → ZnCl2(ac) + 2NH4+(ac)
ZnCl2(ac) + 4NaOH(s) → Na2[ZnO2](ac) + 2NaCl(ac) + 2H2O(l)
Na2[ZnO2](ac) + (NH4)2S(ac) → ↓ZnS(s) + 2NaOH(ac) + 2NH4+(ac)
Cobalto (Co):
Co+2 (ac) + 2NH4OH (ac)) → Co(OH)2 (ac) + 2NH4+(ac)
Co(OH)2 (ac) + (NH4)2S(ac) → ↓CoS(s) + 2NH4OH(ac)
3CoS(s) + 8HNO3(ac) → 3Co(NO3)2 (ac) + ↑2NO(g) + 3S(s) + 4H2O(l)
CH3CH2OCH2CH3
Co(NO3)2 (ac) + 4NH4SCN(s) (NH4)2Co(SCN)4(ac) + 2NH4NO3(ac)
Níquel (Ni):
Ni+2 (ac) + 2NH4OH (ac)) → Ni(OH)2 (ac) + 2NH4+(ac)
Ni(OH)2 (ac) + (NH4)2S(ac) → ↓NiS(s) + 2NH4OH(ac)
3NiS(s) + 8HNO3(ac) → 3Ni(NO3)2 (ac) + ↑2NO(g) + 3S(s) + 4H2O(l)
Ni(NO3)2 (ac) + 2NH4OH(ac) + 2 C4H8O2N2(ac) → (C4H8O2N2)2Ni(ac) + 2NH4NO3(ac) + 2H2O(l)
Manganeso (Mn):
Mn+2 (ac) + 2NH4OH (ac)) → Mn(OH)2(ac) + 2NH4+(ac)
Mn(OH)2 (ac) + (NH4)2S(ac) → ↓MnS(s) + 2NH4OH(ac)
MnS(s) + 2HCl(ac) → Mn+2 (ac) + 2Cl-(ac) + ↑H2S(g)
Mn+2 (ac) + 2NH4Cl(ac) → MnCl2(ac) + 2NH4+(ac)
MnCl2(ac) + 2NaOH(s) + H2O2(l) → ↓MnO(OH)2(s) + 2NaCl(ac) + H2O(l)
2MnO(OH)2(s) + 6HNO3(ac) + 3PbO2(s) → 2HMnO4(ac) + 3Pb(NO3)2(ac) + 4H2O(l)
You might also like
- Reacciones de Arsenio y DemásDocument1 pageReacciones de Arsenio y DemásFernandita Carla CoroNo ratings yet
- Balancing Equations Worksheet Key: ZN (S) + 2 AgnoDocument1 pageBalancing Equations Worksheet Key: ZN (S) + 2 AgnoIgnacio Jr. PaguyoNo ratings yet
- Sap 5Document22 pagesSap 5reza noviyantiNo ratings yet
- NIE - and - Particulate - Drawings - Worksheet - Answers at End - 2017-1Document8 pagesNIE - and - Particulate - Drawings - Worksheet - Answers at End - 2017-1Jane Ivanova100% (1)
- IONIC EQUATIONS AnswersDocument1 pageIONIC EQUATIONS AnswersAlex noslenNo ratings yet
- Chapter 3 Oxidation ReductionDocument68 pagesChapter 3 Oxidation Reductionlong.vuongbz188No ratings yet
- Chem 2Document8 pagesChem 22021302095No ratings yet
- Chapter 4 Oxidation-ReductionDocument68 pagesChapter 4 Oxidation-ReductionPHƯƠNG ĐẶNG YẾNNo ratings yet
- Chemical ReactionsDocument6 pagesChemical ReactionsKushNo ratings yet
- Net Ionic EditedDocument8 pagesNet Ionic EditedMuhammad AbdullahNo ratings yet
- Chem16 Experiment # 1: Chemical Changes Post-Lab Discussion: 3 2 (Aq) (Aq) 2 (S) 3 (Aq) 2+ (Aq) - (Aq) 2 (S)Document1 pageChem16 Experiment # 1: Chemical Changes Post-Lab Discussion: 3 2 (Aq) (Aq) 2 (S) 3 (Aq) 2+ (Aq) - (Aq) 2 (S)DiyanikaNo ratings yet
- ReaccionesDocument3 pagesReaccionesJarek Jhoel Alejandro ZarateNo ratings yet
- Type of Chemical ReactionsDocument3 pagesType of Chemical ReactionsAlex noslenNo ratings yet
- Answer Key To Practice Problems On Net Ionic EquationsDocument4 pagesAnswer Key To Practice Problems On Net Ionic EquationsmerlindikaNo ratings yet
- Francisco Vigil - CHDocument5 pagesFrancisco Vigil - CHapi-528208996No ratings yet
- Edexecel IAL Lesson 1Document20 pagesEdexecel IAL Lesson 1Pevin De silvaNo ratings yet
- Standard Reduction PotentialsDocument3 pagesStandard Reduction PotentialsjaverfrivNo ratings yet
- Reaksi KationDocument9 pagesReaksi KationAnnisa AmaliaNo ratings yet
- CBSE Class 10 Chemistry Worksheet - Chemical Reactions and Equations PDFDocument4 pagesCBSE Class 10 Chemistry Worksheet - Chemical Reactions and Equations PDFMalancha high school HS0% (1)
- CBSE Class 10 Chemistry Worksheet - Chemical Reactions and EquationsDocument4 pagesCBSE Class 10 Chemistry Worksheet - Chemical Reactions and EquationsMalancha high school HS100% (1)
- CBSE Class 10 Chemistry Worksheet - Chemical Reactions and Equations PDFDocument4 pagesCBSE Class 10 Chemistry Worksheet - Chemical Reactions and Equations PDFMalancha high school HS100% (1)
- Chemical Reactions and Equations WS-1Document2 pagesChemical Reactions and Equations WS-1Naman SinghNo ratings yet
- Clasifiacion de Reacciones QuimicasDocument5 pagesClasifiacion de Reacciones QuimicasMONTSERRAT MURILLO SERRANONo ratings yet
- Reaksi KationDocument34 pagesReaksi KationErvina WongsoNo ratings yet
- Chuoi Phan Ung HoaDocument46 pagesChuoi Phan Ung HoaNguyen NgocNo ratings yet
- New Microsoft Word DocumentDocument4 pagesNew Microsoft Word DocumentdalvishreyhansNo ratings yet
- 233 SolutionsDocument11 pages233 Solutionsestellasr00No ratings yet
- Ionic Equations wksht2 PDFDocument2 pagesIonic Equations wksht2 PDFBrandeice Barrett0% (1)
- Standard Reduction PotentialDocument7 pagesStandard Reduction Potentialyoyotoonzone1No ratings yet
- Writing Chemical Equations: Test Yourself 8.1 (Page 130)Document1 pageWriting Chemical Equations: Test Yourself 8.1 (Page 130)Zeeshan MunirNo ratings yet
- Standard Reduction Potentials Data Extended PDFDocument2 pagesStandard Reduction Potentials Data Extended PDFAceNo ratings yet
- Balancing Redox Reactions WorksheetDocument2 pagesBalancing Redox Reactions Worksheetsai sreerama mNo ratings yet
- BALACING CHEM. EQUATIONS AnswersDocument4 pagesBALACING CHEM. EQUATIONS AnswersAlex noslenNo ratings yet
- Chemical Equations: Preparation For College Chemistry Columbia University Department of ChemistryDocument31 pagesChemical Equations: Preparation For College Chemistry Columbia University Department of ChemistryLakshmi SinghNo ratings yet
- Chemical EquationsDocument5 pagesChemical EquationsShweta DharNo ratings yet
- Redoxanswers PDFDocument2 pagesRedoxanswers PDFAlexander Salado IbrahimNo ratings yet
- Redoxanswers PDFDocument2 pagesRedoxanswers PDFalbi veshiNo ratings yet
- Basic Chemical Reaction WorksheetDocument12 pagesBasic Chemical Reaction Worksheettranquil_452889939No ratings yet
- Practice Problems On Net Ionic EquationsDocument3 pagesPractice Problems On Net Ionic EquationsZainabNo ratings yet
- Chemical Equations and Balancing Reactions - KEYDocument1 pageChemical Equations and Balancing Reactions - KEYLam NgọcNo ratings yet
- The Dien Cuc ChuanDocument9 pagesThe Dien Cuc Chuanvinasat1108No ratings yet
- Writing Ionic Equations MSDocument3 pagesWriting Ionic Equations MSSmahs ZabirNo ratings yet
- Chemical Reactions Class XDocument5 pagesChemical Reactions Class Xaprajita royNo ratings yet
- Chemical Reactions in Aqueous SolutionDocument5 pagesChemical Reactions in Aqueous Solutioniam_crazii_4_mhe100% (2)
- Module SaltDocument12 pagesModule SaltAzie Nurul Akhtar100% (1)
- Reaksi Pemisahan KationDocument2 pagesReaksi Pemisahan KationAnisa Nursella TrunodikromoNo ratings yet
- Lesson Plan 5Document15 pagesLesson Plan 5Gusty DyanoNo ratings yet
- C H + 5O 3 Co + 4 H O N (G) + 3 H (G) 2 NH (G) : Setarakan Reaksi Berikut!Document2 pagesC H + 5O 3 Co + 4 H O N (G) + 3 H (G) 2 NH (G) : Setarakan Reaksi Berikut!nurulragilNo ratings yet
- Table of Standard Reduction Potentials (V) +reducing: Half ReactionDocument1 pageTable of Standard Reduction Potentials (V) +reducing: Half ReactionAngates1No ratings yet
- Reaksi Lap 5Document2 pagesReaksi Lap 5YuliaKamilawatiIINo ratings yet
- Molecular Ionic and Net IonicDocument7 pagesMolecular Ionic and Net IonicFarz21No ratings yet
- Molecular Ionic and Net IonicDocument7 pagesMolecular Ionic and Net IonicFarz21No ratings yet
- 110 WS Net Ionic Eqns KeyDocument3 pages110 WS Net Ionic Eqns KeyZaara RyeenNo ratings yet
- C10 Chem Holiday AssignmentDocument4 pagesC10 Chem Holiday AssignmentRaj DulariNo ratings yet
- Chem 110 Quiz BalancingDocument1 pageChem 110 Quiz BalancinglucasNo ratings yet
- 121Document1 page121Markus AbiogNo ratings yet
- Unit 4 Formulae and Equations: Summary QuestionsDocument2 pagesUnit 4 Formulae and Equations: Summary QuestionsLei YinNo ratings yet
- Problems For Balancing of Redox ReactionsDocument1 pageProblems For Balancing of Redox ReactionsUtsavNo ratings yet
- Worksheet 1 Chemistry F4 Chapter3 Chemical Equation AnswerDocument2 pagesWorksheet 1 Chemistry F4 Chapter3 Chemical Equation AnswerIpul Catur0% (1)
- Unusual Structures and Physical Properties in Organometallic ChemistryFrom EverandUnusual Structures and Physical Properties in Organometallic ChemistryNo ratings yet