Professional Documents
Culture Documents
Frozen & Aseptic Mango Puree
Uploaded by
Domel Jhotsmer VasquezOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Frozen & Aseptic Mango Puree
Uploaded by
Domel Jhotsmer VasquezCopyright:
Available Formats
MANGO PUREE ASEPTIC
GENERAL SPECIFICATIONS
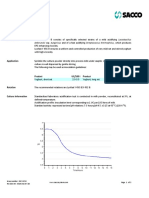
HARVEST
J F M A M
ariety: Manguifera indica • •
Origin: Ecuador / Perú. J A S O N D
• •
DESCRIPTIONS AND USES
Our Puree is obtained from sound, ripe and unfermented fruit by
mechanical processes and is solely made from the named fruit and it TRANSPORT CONDITIONS
contains no other additive, coloring, sugar and preservatives then Deep frozen at -18ºC / Aseptic product at ambient, avoid contact with
mentioned. sunlight, and keep in a cool place preferably not exceeding 25 C..
The Mango Puree is intended to be used as base for production of fruit
juice, beverage and wine as well as additive to other foodstuff SHELF LIFE CONDITIONS
FROZEN 24 months from production date. Stored
CHEMICAL AND PHYSICAL CHARACTERISTICS frozen at -15°C to – 18°C. To consume
Brix uncorr Min14 IFFJP Nº8 1991 immediately after having defrosted
Ph 3.7 – 4.3 IFFJP Nº11 1989
Acidity (ACA) 0.30– Mettler Toledo T50 12 months from production date.
Pulp 0.45% Internal Method Acceptable temperature 20°C to 30°C.
60 – 98% ASEPTIC Avoid direct sunlight exposure
18 months from production date. Optimal
temperature < 20°C. Avoid direct sunlight
exposure.
SPECIFICATION METHOD
TYPICAL FROZEN ASEPTIC After 6 months, to preserve top color and freshness for up to 18
REFEREN
MICROBIOLOGY months, keep chilled at +6C. For longer storage freezing is suggested
CE
*TVC <1000 cfu/g <10 cfu/g BAM 2001
*Yeast <500 cfu/g <10 cfu/g BAM 2001 Parameters maintained if kept at proper shelf life conditions as stated
*Mold <100 cfu/g <10 cfu/g BAM 2001 in this spec.
E.coli 0157:h7 absent/25g absent/25g AOAC 2000.13
Total coliforms absent absent Petrifilm AOAC
Listeria spp. abs/25g - Reveal AOAC AUTHENTICITY
Salmonella abs/25g - Reveal AOAC The products is made from 100% natural, fresh and wholesome fruit of
*TAB 0/50 cfu/g 0/50 cfu/g NFPA/APHA the specified varieties and is not modified or adulterated in any way by
*HRM 0/50 cfu/g 0/50 cfu/g NFPA/APHA addition of food additives or unauthorized processing.
Anaerobic bacteria Absent BAM 2001 Our product complies with the Directive 2001/112/EC complies
*According to AIJN /10g furthermore with the AIJN Code of practice. Genetic manipulated fruit
is not used.
ORGANOLEPTIC CHARACTERISTICS
Aspect Viscous, viscous Visual PROCESS DESCRIPTION AND HYGIENE
The total process (all steps of production) is according to the norm
Yellow to Yellow to FSSC22000, HACC, GMP and Codex Alimentarius Commission, FDA
Color Bright Orange Pantone 2009 Food Code or 852/2004 EC and subsequent amendments.
Orange
Aroma & flavor Aromatic Aromatic FOOD ADDITIVES
fresh fruit strong fruity IFJU Nº25 Ascorbic and /or citric added up to 3ppm.
The product does not contain any food additives. Processing aids as
TYPE AND PACKAGING UNIT far authorized by the directive 2001/112/EC have not been used or
21CFR170-189.
Primary: Double polybags each
Drums individually tied. PACKING
(200 kg) Secondary: Open-head steel drums for The packing has to be uniform. The packaging materials have to
FROZEN foodstuff use. comply with the requirements of the relevant version of the regulation
Primary: Double polybags each (EC) N°1935/2004 and its implementation measures.
Pail
(18.5 kg) individually tied.
Secondary: Open-head plastic pail. CONTAMINANTS
In mg/l reconstructed juice at 14 Brix.
Primary: Double bag (Polyethylene The values for heavy metal should not exceed the limits AIJN Code of
Drums and Aseptic). Practices and accordingly commission regulation (EC) N°1881/2006.
(220 kg) Secondary: Open-head steel drums for
ASEPTIC foodstuff use.
RESIDUES PESTICIDES
Primary: Aseptic bag.
Bag-in-box In mg/l reconstructed juice at 14 Brix.
Secondary: Carton bag-in-box (Kraft –
(22kg) During cultivations only pesticides and agro – chemicals approved in
flute C/B) EC were used and the residues do not exceed the maximum levels of
the regulation (EC) N° 396/2005 as amended, Environmental
LABELING Protection Agency ( EPA) 21CFR179.19
Labels contain following data: According to AIJ N or 21CFR101
DISCLAIMER
- Producer - Traceability data The values and parameters as detailed in the current general
- Product name - Storage specifications are the result of 1) validation per batch 2) yearly food safety
system validation. While we do our best to assure that all our supplied
- Product code - Production date
products fall within the local juice legislation, it is the sole responsibility of
- Brix - Net weight the end user to ultimately ensure that safety, residues, organoleptic and
- ingredient others sensory-analytical values of the supplied product fall within an
approved range before using this product. Prom Commerce SA/SAC does
not accept any liability or claims arising from the use of our products,
which do not meet approval criteria for use or do not fall within accepted
ranges according to Food Law and its Directives. In case of a claim,
exchange or refund of product can be provided subject to availability, if we
are notified before use, and that the product is in its original state and
packaging and that a third party lab can verify that the product does not
meet the values that allow its marketability for use in the juice industry.
This product has been manufactured in accordance with Food Safety System certification FSSC 22000
You might also like
- Telescopic Handler Student ManualDocument41 pagesTelescopic Handler Student ManualingcalderonNo ratings yet
- MSDS Antifoam Silicone Base 1520-USDocument3 pagesMSDS Antifoam Silicone Base 1520-USMarlufi SulindraNo ratings yet
- Sarika RobinsonDocument5 pagesSarika Robinsonapi-355030459No ratings yet
- Pectinase 831LDocument1 pagePectinase 831LVeronica V. PortillaNo ratings yet
- TDS 2932 Maca Powder V 09-2017Document3 pagesTDS 2932 Maca Powder V 09-2017lisetteNo ratings yet
- Maca Powder Organic V 10-2017Document3 pagesMaca Powder Organic V 10-2017Marcos Rolando HrNo ratings yet
- TDS - 4941 - Purple Corn Extract 12.1 V10-2021Document3 pagesTDS - 4941 - Purple Corn Extract 12.1 V10-2021cristianNo ratings yet
- Garlic FlakesDocument1 pageGarlic Flakesedward120703No ratings yet
- Banana PulpDocument5 pagesBanana PulpTejas ShriyanNo ratings yet
- Agchem Al 5 LitreDocument1 pageAgchem Al 5 LitrehardiksurtiNo ratings yet
- Garlic GranulesDocument1 pageGarlic Granulesedward120703No ratings yet
- Garlic PowderDocument1 pageGarlic Powderedward120703No ratings yet
- TDS - Saffron Strands Iso IiDocument3 pagesTDS - Saffron Strands Iso IiJasvinder SethiNo ratings yet
- Apple Dices-Without SkinDocument1 pageApple Dices-Without Skinedward120703No ratings yet
- Ficha Tecnica Divalash2Document1 pageFicha Tecnica Divalash2Paula CeledonNo ratings yet
- AloeVeraOil COA 1602180318Document2 pagesAloeVeraOil COA 1602180318Abdullahil KafiNo ratings yet
- Biolife: Nutrient AgarDocument2 pagesBiolife: Nutrient AgarZoza SalamaNo ratings yet
- P & G ChemicalsDocument1 pageP & G ChemicalsRikardo LumbantoruanNo ratings yet
- Merck Rebrand - 100908 - 1907Document5 pagesMerck Rebrand - 100908 - 1907Paula BautistaNo ratings yet
- Myristate-Disopropyle RSPO 2022maiDocument3 pagesMyristate-Disopropyle RSPO 2022maihadjer djemiliNo ratings yet
- Certificate of Analysis (Coa) : Aerobic Mesophilic Bacterial CountDocument2 pagesCertificate of Analysis (Coa) : Aerobic Mesophilic Bacterial CountDima ArfNo ratings yet
- Bioligo GL-5700 IMF (C) - 70000307 - ENDocument2 pagesBioligo GL-5700 IMF (C) - 70000307 - ENtanya.decastro17No ratings yet
- TDS - Saffron Strands Iso IiiDocument3 pagesTDS - Saffron Strands Iso IiiJasvinder SethiNo ratings yet
- Specifications: Production Process and Quality Management SystemDocument3 pagesSpecifications: Production Process and Quality Management SystemKalpesh RathodNo ratings yet
- Agua de Peptona Tamponada MerckDocument5 pagesAgua de Peptona Tamponada MerckLuz Katherine MartinezNo ratings yet
- Technical Data Sheet For Instant Dry Baker'S YeastDocument1 pageTechnical Data Sheet For Instant Dry Baker'S YeastRakesh SakarayNo ratings yet
- Grapefruit Essential Oil COA 10812305Document2 pagesGrapefruit Essential Oil COA 10812305nadyaNo ratings yet
- Upl 60705c37ec35fDocument2 pagesUpl 60705c37ec35fLUZ RAMOSNo ratings yet
- Certificate of Analysis (Coa) : Aerobic Mesophilic Bacterial CountDocument2 pagesCertificate of Analysis (Coa) : Aerobic Mesophilic Bacterial Countrand0No ratings yet
- Product Data Sheet: Novamyl® 1500 MGDocument2 pagesProduct Data Sheet: Novamyl® 1500 MGمحمد صبحيNo ratings yet
- Steranios 20 Concentre DS-AEDocument188 pagesSteranios 20 Concentre DS-AEalexa1715No ratings yet
- TDS TrehaloseDocument2 pagesTDS TrehaloseArthur SaadaNo ratings yet
- UntitledDocument6 pagesUntitledIssam LahlouNo ratings yet
- Guava PulpDocument4 pagesGuava PulpTejas ShriyanNo ratings yet
- Cassie Absolute Oil TDSDocument2 pagesCassie Absolute Oil TDSAlbertoNo ratings yet
- Eltex B4020N1332: Product Technical InformationDocument2 pagesEltex B4020N1332: Product Technical InformationAndres Giovanni Romero MuñozNo ratings yet
- CN Agar PseudomonasDocument5 pagesCN Agar PseudomonasPaulina RosaNo ratings yet
- Specs - MInced GarlicDocument2 pagesSpecs - MInced GarlicAmparo BagnisalNo ratings yet
- Lacprodan Clear Shake P1279Document2 pagesLacprodan Clear Shake P1279Alexandre FarrantNo ratings yet
- N-Zorbit 2144 DG - enDocument3 pagesN-Zorbit 2144 DG - enfernando guzman garciaNo ratings yet
- Uma Co., Ltd. Hba1C Diluent: 2-19-6 Yokosuka Matsudo, Chiba, JapanDocument1 pageUma Co., Ltd. Hba1C Diluent: 2-19-6 Yokosuka Matsudo, Chiba, JapanTrần Văn BìnhNo ratings yet
- Product Spec Pineapple PureeDocument2 pagesProduct Spec Pineapple PureeDelvi AddeliaNo ratings yet
- 21 07 22 Y456bDocument3 pages21 07 22 Y456bYogures ZhuaNo ratings yet
- Updated On: Descriptive SynopsisDocument3 pagesUpdated On: Descriptive SynopsisSANo ratings yet
- General Information: Product Technical Data SheetDocument3 pagesGeneral Information: Product Technical Data SheetOKACI NAZIDNo ratings yet
- Superol Kpo Tds 2012Document1 pageSuperol Kpo Tds 2012rusyadNo ratings yet
- 156-Skimmed Milk Medium Heat - Rev 1a - GBDocument2 pages156-Skimmed Milk Medium Heat - Rev 1a - GBMostafa AlakhliNo ratings yet
- Agarwood (Oud) Essential Oil COA 12212309Document2 pagesAgarwood (Oud) Essential Oil COA 12212309sm9s4jxkkbNo ratings yet
- Carrot Flakes ADocument1 pageCarrot Flakes Aedward120703No ratings yet
- Fichas Tecnicas - Derivados Del CacaoDocument4 pagesFichas Tecnicas - Derivados Del CacaoGabrielaNo ratings yet
- Yo-Mix 465 Lyo 200 Dcu - PDDocument2 pagesYo-Mix 465 Lyo 200 Dcu - PDHuyền Trần100% (1)
- Granucult™ Mrs Agar (De Man, Rogosa and Sharpe) Acc. Iso 15214Document4 pagesGranucult™ Mrs Agar (De Man, Rogosa and Sharpe) Acc. Iso 15214Yanick VeraNo ratings yet
- Specification: General InformationDocument3 pagesSpecification: General InformationAnna Carolina FerreiraNo ratings yet
- Almidon Cargilltex BlendtechDocument4 pagesAlmidon Cargilltex Blendtechinnovacionydesarrollo SuplacolNo ratings yet
- Macconkey Agar Macconkey Agar: DescriptionDocument2 pagesMacconkey Agar Macconkey Agar: DescriptionKATHENo ratings yet
- IQF Pineapple Dices PDFDocument3 pagesIQF Pineapple Dices PDFMynthan MoorthyNo ratings yet
- SE73255 Product DeclarationDocument3 pagesSE73255 Product DeclarationJon BernasconiNo ratings yet
- COLUMBIA Lable OrgofertDocument5 pagesCOLUMBIA Lable OrgofertMarcelo ViscardiNo ratings yet
- Specs 10-12 QUEEN DNG71Document5 pagesSpecs 10-12 QUEEN DNG71ABCO SA de CVNo ratings yet
- Ficha Tecnica Indicador Biologico BT20Document4 pagesFicha Tecnica Indicador Biologico BT20CATALINA FAJARDONo ratings yet
- Approval Romania 2008Document27 pagesApproval Romania 2008Andrés Domínguez RuizNo ratings yet
- Science3 Q1 W4 Proper Use HandlingDocument50 pagesScience3 Q1 W4 Proper Use HandlingGina Contalba TubeoNo ratings yet
- Minimizing The Risk of Alzheimer S DiseaseDocument328 pagesMinimizing The Risk of Alzheimer S DiseaseLuis Raudales100% (1)
- (OSHPD 1, 2, 3 & 4) See Sections 404.0 Through 418.0. (SFM) Air Filters Shall Comply With All Requirements ofDocument0 pages(OSHPD 1, 2, 3 & 4) See Sections 404.0 Through 418.0. (SFM) Air Filters Shall Comply With All Requirements ofOanh NguyenNo ratings yet
- Power2 340-H44: Operation ManualDocument166 pagesPower2 340-H44: Operation ManualDmitrii PustoshkinNo ratings yet
- Ims 1 10 18Document2 pagesIms 1 10 18Katherine UrregoNo ratings yet
- Rhomboid Flap For Pilonidal Sinus - Our ExperienceDocument5 pagesRhomboid Flap For Pilonidal Sinus - Our ExperienceKhalidHussainNo ratings yet
- Drug Dispensing Practices at Pharmacies in Bengaluru: A Cross Sectional StudyDocument5 pagesDrug Dispensing Practices at Pharmacies in Bengaluru: A Cross Sectional StudySoumya RatnalaNo ratings yet
- Nepal New Emerging Pharma MarketDocument5 pagesNepal New Emerging Pharma MarketDeep_HeartNo ratings yet
- Are You Planning To Buy or Rent A Home Built Before 1978?Document60 pagesAre You Planning To Buy or Rent A Home Built Before 1978?Maria FloresNo ratings yet
- APSAC FI Guidelines 2012Document28 pagesAPSAC FI Guidelines 2012gdlo72No ratings yet
- Earth Fare PresentationDocument36 pagesEarth Fare Presentationapi-253275458No ratings yet
- Hba1C: Quality System CertifiedDocument4 pagesHba1C: Quality System CertifiedNonameNo ratings yet
- Liver Vascular Anatomy - A RefresherDocument10 pagesLiver Vascular Anatomy - A Refresherilham nugrohoNo ratings yet
- Career Planning WorkbookDocument41 pagesCareer Planning WorkbookEmilyNo ratings yet
- Second Circuit Public Charge RulingDocument110 pagesSecond Circuit Public Charge RulingLaw&CrimeNo ratings yet
- Essential Nutrients - Minerals: 6.1 Introduction and ClassificationDocument15 pagesEssential Nutrients - Minerals: 6.1 Introduction and ClassificationRavinder RanaNo ratings yet
- Cannabis-Infused Food and Canadian Consumers' Willingness To Consider "Recreational" Cannabis As A Food IngredientDocument7 pagesCannabis-Infused Food and Canadian Consumers' Willingness To Consider "Recreational" Cannabis As A Food IngredientCTV CalgaryNo ratings yet
- Effect of Human Papilloma Virus in HIV Infected Person: A Mini ReviewDocument7 pagesEffect of Human Papilloma Virus in HIV Infected Person: A Mini ReviewKIH 20162017No ratings yet
- New Text DocumentDocument2 pagesNew Text DocumenteqweNo ratings yet
- Transdiagnostic TreatmentDocument9 pagesTransdiagnostic Treatmentvalentina chistrugaNo ratings yet
- Circadian RhythmsDocument2 pagesCircadian RhythmsOmar Saleh100% (1)
- Muet EssaysDocument29 pagesMuet EssaysBabasChongNo ratings yet
- Valid RRR Application 2013 - 2017 PDFDocument22,467 pagesValid RRR Application 2013 - 2017 PDFAyub NaveedNo ratings yet
- Policy That Implemented in Kota SamarahanDocument9 pagesPolicy That Implemented in Kota SamarahanIskandar IskandarNo ratings yet
- Complete Holistic Guide To Working Out in The GymDocument218 pagesComplete Holistic Guide To Working Out in The Gympsichi21No ratings yet
- Alice in Michigan: A Financial Hardship StudyDocument58 pagesAlice in Michigan: A Financial Hardship StudydaneNo ratings yet
- MAPEH Activity Sheet Q2-Week1Document7 pagesMAPEH Activity Sheet Q2-Week1John King johnking.monderinNo ratings yet