Professional Documents
Culture Documents
Zinc Sulfide Topical Suspension, USP, Lotio Alba, Lotio Sulfurata Zinc Sulphide
Uploaded by
Joanna Carla Marmonejo Estorninos-WalkerOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Zinc Sulfide Topical Suspension, USP, Lotio Alba, Lotio Sulfurata Zinc Sulphide
Uploaded by
Joanna Carla Marmonejo Estorninos-WalkerCopyright:
Available Formats
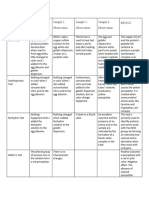
EXPERIMENT NO.
11 PREPARATION OF WHITE LOTION
Synonyms: Zinc Sulfide Topical Suspension, USP, Lotio Alba, Lotio Sulfurata
Active component: Zinc sulphide Physical Test
Zn+2: Astringent and protective action Color White
Thiosulfate and polysulfides: Antibacterial and antifungal activity Odor Odorless
Pharm use of White Lotion: Physical Liquid
used as antipruritic and used to relieve itching and pain of sunburn, insect bites and State
other minor irritations, esthetic and antiseptic treatment of acne vulgaris.
Astringent which may have several benefits in your skin.
Also a protective agent and a mild antimicrobial preparation.
Chemical Test
Test for Potassium precipitation with potassium ions which White lotion USP solution or sulfurated potash solution
ion is potassium nitrite precipitation. added with sodium cobalt nitrite will form precipitation
White lotion or It is an ionic salt of potassium ions and with potassium ions which is potassium nitrite precipitation.
sulfureted potash + nitrite ions, which forms a white or It is an ionic salt of potassium ions and nitrite ions, which
Sodium cobalt slightly or pale yellow hygroscopic forms a white or slightly or pale yellow hygroscopic
nitrate crystalline powder crystalline powder that is soluble in water
Test for Sulfide ion color of the lotion or sulfurated potash As the white lotion and 6N sulfuric acid is mixed and
White lotion + will then transfer to the filter paper. covered by watch glass with filter paper moistened with
Sulfuric acid lead acetate, the color of the lotion or sulfurated potash
will then transfer to the filter paper. Sulfide colors can be
yellow, brown and black.
The nitroprusside reaction is a chemical test used to detect
the presence of thiol groups of cysteine in proteins. As the
sodium nitroprusside solution is dropped to the white
lotion, the free thiol group will give a red color.
Test for Zinc ion Precipitate of zinc sulfide Zinc sulfate from the solution of sulturated potash will react
Zinc sulfate + with ammonium sulfide to form a precipitate of zinc
ammonium sulfide sulfide. This zinc sulfide is white and transparent in color
Flame test for Purple flame Purple flame was observed or produced using the non-
Potassium Crimson red flame luminous flame.
Crimson red flame using cobalt glass
You might also like
- Post TestsDocument37 pagesPost TestsRobby ZablanNo ratings yet
- Acid Base & SaltsDocument7 pagesAcid Base & SaltsAnonymous BnZrzImmC9No ratings yet
- Mls-5a Bsmls2-E Module4 Group9 NacionalesDocument11 pagesMls-5a Bsmls2-E Module4 Group9 NacionalesLexi Evonne NacionalesNo ratings yet
- MLS 5a - BSMLS2-E - Module4 - Group9Document8 pagesMLS 5a - BSMLS2-E - Module4 - Group9Lexi Evonne NacionalesNo ratings yet
- Chem Procedure-Inorg Salt - 2021Document9 pagesChem Procedure-Inorg Salt - 2021S3er IgNo ratings yet
- Salt Procedure For 23-24Document4 pagesSalt Procedure For 23-24arshdeep.kaur1ejNo ratings yet
- Radl Week 1Document36 pagesRadl Week 1Zeian Jacob BaylaNo ratings yet
- Type of TestDocument3 pagesType of TestNikko ReyesNo ratings yet
- Pactical 2 - Amino Acid and ProteinDocument5 pagesPactical 2 - Amino Acid and ProteinlimNo ratings yet
- Salt AnalysisDocument4 pagesSalt Analysisnitheeshchowdary2007No ratings yet
- Chem7a BSN-1-J Module4Document5 pagesChem7a BSN-1-J Module4Kiana JezalynNo ratings yet
- Chem41 Postlabexpt.n0.3Document36 pagesChem41 Postlabexpt.n0.3HJakansjakkaNo ratings yet
- Tamil Nadu Board Class 11 - Chemistry Practical BookDocument9 pagesTamil Nadu Board Class 11 - Chemistry Practical BookAjith KumarNo ratings yet
- Practical Final 3-4-2014Document11 pagesPractical Final 3-4-2014azadbashaNo ratings yet
- Analysis of Salts: Physical ExaminationDocument5 pagesAnalysis of Salts: Physical ExaminationSuchir PatNo ratings yet
- Chemistry Practical Help For XiiDocument16 pagesChemistry Practical Help For XiiMehjabin Abdurrazaque50% (8)
- Color Reactions of Proteins and Amino AcidsDocument7 pagesColor Reactions of Proteins and Amino AcidsFARHANA ASDAIN INJAN100% (1)
- Module 10 Lab ReportDocument5 pagesModule 10 Lab ReportVon Nilshen IlejayNo ratings yet
- Hsslive XII Chemistry Practical Salt - Analysis - EngDocument3 pagesHsslive XII Chemistry Practical Salt - Analysis - EngNandaj Manu100% (3)
- BiochemDocument3 pagesBiochemPaulene Marie SicatNo ratings yet
- Experiment No. 2 - ClaritoDocument4 pagesExperiment No. 2 - ClaritoQuincy Mary ClaritoNo ratings yet
- Biochem LabDocument24 pagesBiochem Lab813 cafeNo ratings yet
- 1 Salt Analysis Lead AcetateDocument2 pages1 Salt Analysis Lead AcetateSuman PandeyNo ratings yet
- VI. AnalysisDocument5 pagesVI. AnalysisAdrian Alvinson NazarenoNo ratings yet
- M6 La1-Post Task Activity Sheet (Laboratory)Document5 pagesM6 La1-Post Task Activity Sheet (Laboratory)Mary Joeh LlarenaNo ratings yet
- Solution Observatio N Interpretation 1% Albumin 1% Casein 1% Peptone Solution Observation Interpretation Urea With Distilled WaterDocument8 pagesSolution Observatio N Interpretation 1% Albumin 1% Casein 1% Peptone Solution Observation Interpretation Urea With Distilled WatershakesNo ratings yet
- P2. Laboratory Exam. Part 1: Test For Amino AcidsDocument3 pagesP2. Laboratory Exam. Part 1: Test For Amino AcidsAllejah Jane CantaNo ratings yet
- Color Reaction of ProteinsDocument28 pagesColor Reaction of ProteinsKae ArturiaNo ratings yet
- Formal Report CaseinDocument5 pagesFormal Report CaseinBianca Ocampo100% (3)
- Lab 2 - Org ChemDocument11 pagesLab 2 - Org Chemchris andrieNo ratings yet
- Chemistry - Lead Nitrate - Lab ProcedureDocument3 pagesChemistry - Lead Nitrate - Lab Procedurejackiepuck11No ratings yet
- Iodimetry and IodometryDocument6 pagesIodimetry and IodometrythereseNo ratings yet
- XI Chemistry Practical Guide EM (325 Students)Document30 pagesXI Chemistry Practical Guide EM (325 Students)ppowjiyaNo ratings yet
- Chemical Tests 1Document1 pageChemical Tests 1sara.u1925No ratings yet
- Expt 6Document10 pagesExpt 6beatriz balingit0% (1)
- Biochem Post Lab 4bDocument7 pagesBiochem Post Lab 4bJessica Lorenz PablicoNo ratings yet
- Exp 2 Formal ReportDocument50 pagesExp 2 Formal ReportNatalie CuNo ratings yet
- Experiment 4Document4 pagesExperiment 4jamielNo ratings yet
- URINE EXMN With AnswersDocument12 pagesURINE EXMN With AnswersSivaani ChidambaramNo ratings yet
- Analysis of Salts: Experiment Observation InferenceDocument3 pagesAnalysis of Salts: Experiment Observation InferenceSuchir PatNo ratings yet
- Nucleic AcidDocument34 pagesNucleic AcidEinah EinahNo ratings yet
- Salt AnalysisDocument8 pagesSalt AnalysisB.K.Sivaraj raj0% (1)
- Hsslive XII Chemistry Practical Salt - Analysis - Eng 1 PDFDocument3 pagesHsslive XII Chemistry Practical Salt - Analysis - Eng 1 PDFaromalssatheesh02No ratings yet
- Salt Analysis-Ferric ChlorideDocument3 pagesSalt Analysis-Ferric ChlorideVandana0% (1)
- Color Reactions of Casein Protein and HydrolysateDocument6 pagesColor Reactions of Casein Protein and HydrolysateBianca OcampoNo ratings yet
- Exp 2Document4 pagesExp 2EmmyNo ratings yet
- Dechavez 2e Experiment3Document6 pagesDechavez 2e Experiment3Jhes D.No ratings yet
- Biochemistry Laboratory 789456123Document3 pagesBiochemistry Laboratory 789456123clienteditingaccNo ratings yet
- M4 Check in ActivityDocument2 pagesM4 Check in Activityjelly fishNo ratings yet
- Copper Sulphate 10Document2 pagesCopper Sulphate 10C. SathisNo ratings yet
- Group Iv Tests: Acetylation TestDocument7 pagesGroup Iv Tests: Acetylation TestAnanda VijayasarathyNo ratings yet
- Post Lab QuestionDocument3 pagesPost Lab QuestionJoshua DividinaNo ratings yet
- Experiment 1 Preparation and Standardization of Volumetric SolutionsDocument28 pagesExperiment 1 Preparation and Standardization of Volumetric SolutionsMylene Mendoza0% (2)
- S.No - Experiment Observation Inference: Systematic Analysis of Inorganic Salt Mixture - IiDocument7 pagesS.No - Experiment Observation Inference: Systematic Analysis of Inorganic Salt Mixture - IiArchana ArchuNo ratings yet
- IIB Esmaya Jessalyn FinalDocument17 pagesIIB Esmaya Jessalyn FinalJuliet T. EsmayaNo ratings yet
- Qualitative Tests On Amino Acids and ProteinsDocument9 pagesQualitative Tests On Amino Acids and ProteinsCorine RepatoNo ratings yet
- Chem (Acids, Bases & Salts Basics) SveaDocument6 pagesChem (Acids, Bases & Salts Basics) Svearbkia470No ratings yet
- Qualitative Analysis of Inorganic Salt 11 and 12 ChemistryDocument66 pagesQualitative Analysis of Inorganic Salt 11 and 12 Chemistrybakhshishh06No ratings yet
- Systematic Organic AnalysisDocument6 pagesSystematic Organic Analysisapi-19520338100% (5)
- Extraction and Identification of CarbohydrateDocument7 pagesExtraction and Identification of CarbohydrateJoanna Carla Marmonejo Estorninos-WalkerNo ratings yet
- Isolation of Citric AcidDocument6 pagesIsolation of Citric AcidJoanna Carla Marmonejo Estorninos-Walker100% (1)
- Definition of GlobalizationDocument7 pagesDefinition of GlobalizationJoanna Carla Marmonejo Estorninos-WalkerNo ratings yet
- Important TermsDocument18 pagesImportant TermsJoanna Carla Marmonejo Estorninos-WalkerNo ratings yet
- ITOM, PUTI Pareha Lang TA!Document1 pageITOM, PUTI Pareha Lang TA!Joanna Carla Marmonejo Estorninos-WalkerNo ratings yet
- Organoleptic Evaluation (Leaves)Document19 pagesOrganoleptic Evaluation (Leaves)Joanna Carla Marmonejo Estorninos-WalkerNo ratings yet
- Healthy Hearts Write UpDocument1 pageHealthy Hearts Write UpJoanna Carla Marmonejo Estorninos-WalkerNo ratings yet
- Equal Pay ActDocument2 pagesEqual Pay ActJoanna Carla Marmonejo Estorninos-WalkerNo ratings yet
- Classwork 3 - ESTORNINOSDocument2 pagesClasswork 3 - ESTORNINOSJoanna Carla Marmonejo Estorninos-WalkerNo ratings yet
- Globalization and ReligionDocument2 pagesGlobalization and ReligionJoanna Carla Marmonejo Estorninos-Walker0% (1)
- Define Globalization and Expound Your Answer in No Less Than Five (5) SentencesDocument10 pagesDefine Globalization and Expound Your Answer in No Less Than Five (5) SentencesJoanna Carla Marmonejo Estorninos-WalkerNo ratings yet
- GlobalizationDocument6 pagesGlobalizationJoanna Carla Marmonejo Estorninos-WalkerNo ratings yet
- Classwork 3 - ESTORNINOSDocument2 pagesClasswork 3 - ESTORNINOSJoanna Carla Marmonejo Estorninos-WalkerNo ratings yet
- Biochem Finals CTRL FDocument11 pagesBiochem Finals CTRL FJoanna Carla Marmonejo Estorninos-WalkerNo ratings yet
- 1 Introduction To Pharmaceutical Dosage Forms Part1Document32 pages1 Introduction To Pharmaceutical Dosage Forms Part1Joanna Carla Marmonejo Estorninos-Walker100% (1)
- Finals Review Biochem Lab CTRL FDocument5 pagesFinals Review Biochem Lab CTRL FJoanna Carla Marmonejo Estorninos-WalkerNo ratings yet
- 3 The 7star Pharmacist Concept by WHODocument19 pages3 The 7star Pharmacist Concept by WHOJoanna Carla Marmonejo Estorninos-WalkerNo ratings yet
- 6 Pharmacists in Public HealthDocument22 pages6 Pharmacists in Public HealthJoanna Carla Marmonejo Estorninos-Walker100% (1)
- Alcohol Product/ Synonyms IUPAC Name Method of Preparation Classification/Properti Es Uses 1. Methyl Alcohol - Wood SpiritDocument8 pagesAlcohol Product/ Synonyms IUPAC Name Method of Preparation Classification/Properti Es Uses 1. Methyl Alcohol - Wood SpiritJoanna Carla Marmonejo Estorninos-WalkerNo ratings yet
- 3 The 7star Pharmacist Concept by WHODocument19 pages3 The 7star Pharmacist Concept by WHOJoanna Carla Marmonejo Estorninos-WalkerNo ratings yet
- 5 Pharmacy Practice in IndustryDocument25 pages5 Pharmacy Practice in IndustryJoanna Carla Marmonejo Estorninos-WalkerNo ratings yet
- 1 EssentialsDocument4 pages1 EssentialsJoanna Carla Marmonejo Estorninos-WalkerNo ratings yet
- 1 Historical Background of Pharmacy Profession (Autosaved)Document43 pages1 Historical Background of Pharmacy Profession (Autosaved)Joanna Carla Marmonejo Estorninos-WalkerNo ratings yet
- Answer: 4-Methyl-2-PenteneDocument3 pagesAnswer: 4-Methyl-2-PenteneJoanna Carla Marmonejo Estorninos-WalkerNo ratings yet
- Special Senses: Part I: The Eye and VisionDocument8 pagesSpecial Senses: Part I: The Eye and VisionJoanna Carla Marmonejo Estorninos-WalkerNo ratings yet
- Skin and Body MembranesDocument7 pagesSkin and Body MembranesJoanna Carla Marmonejo Estorninos-Walker100% (2)
- Peripheral Neuropathy Myelosuppression Renal Impairment Gastrointestinal Disturbances Together With Changes in TasteDocument4 pagesPeripheral Neuropathy Myelosuppression Renal Impairment Gastrointestinal Disturbances Together With Changes in TasteJoanna Carla Marmonejo Estorninos-WalkerNo ratings yet
- 1 PetroroleumDocument9 pages1 PetroroleumJoanna Carla Marmonejo Estorninos-WalkerNo ratings yet
- Meals: Abbrev. Meaning (Or) OriginDocument5 pagesMeals: Abbrev. Meaning (Or) OriginJoanna Carla Marmonejo Estorninos-WalkerNo ratings yet
- FUNGIDocument8 pagesFUNGIJoanna Carla Marmonejo Estorninos-WalkerNo ratings yet
- CHM 3202 Lab#2Document13 pagesCHM 3202 Lab#2Raja GokhulNo ratings yet
- Module Chem F4 Chapter 2-8Document29 pagesModule Chem F4 Chapter 2-8CikFasyareena MaoNo ratings yet
- Producing Exactly 2.00 Grams of A Compound Lab MSDSDocument2 pagesProducing Exactly 2.00 Grams of A Compound Lab MSDSMichael Kevin YangNo ratings yet
- Solubility Product Constant of Lead (II) Chloride C12-4-13Document6 pagesSolubility Product Constant of Lead (II) Chloride C12-4-13shayneNo ratings yet
- Chemistry Jun 2010 Actual Exam Paper Unit 6Document16 pagesChemistry Jun 2010 Actual Exam Paper Unit 6dylandonNo ratings yet
- Waste Water TreatmentDocument43 pagesWaste Water TreatmentRahul Deka100% (1)
- FHSC1134 Lab Manual V4 2-1Document29 pagesFHSC1134 Lab Manual V4 2-1GOUK SY KAI KELVINNo ratings yet
- Alchemists ConcordanceDocument108 pagesAlchemists Concordanceroger santosNo ratings yet
- Chemistry Study Notes Igcse EdexcelDocument34 pagesChemistry Study Notes Igcse EdexcelAhmed KhalilNo ratings yet
- Easy Caapi Vine Alkaloid Extraction GuideDocument5 pagesEasy Caapi Vine Alkaloid Extraction GuiderozeNo ratings yet
- Analyse Organic and Inorganic Unknowns WORDDocument5 pagesAnalyse Organic and Inorganic Unknowns WORDcydney mackenzieNo ratings yet
- Themochemistry NotesDocument26 pagesThemochemistry NotesjayaselanNo ratings yet
- CHM 123L Lab 1 Due September 9, 2013 Gravimetric Analysis of Phosphorus in Plant FoodDocument6 pagesCHM 123L Lab 1 Due September 9, 2013 Gravimetric Analysis of Phosphorus in Plant FoodAhmed IsmailNo ratings yet
- GravimetryDocument15 pagesGravimetryAura Paige Montecastro-RevillaNo ratings yet
- Instant Download Design of Wood Structures Asd LRFD 6th Edition Donald Breyer Solutions Manual PDF Full ChapterDocument32 pagesInstant Download Design of Wood Structures Asd LRFD 6th Edition Donald Breyer Solutions Manual PDF Full Chaptermikadischerpro90% (10)
- Codex 2017 enDocument713 pagesCodex 2017 enJIgnacio123No ratings yet
- Chemical Analysis of Red Lead: Standard Test Methods ofDocument4 pagesChemical Analysis of Red Lead: Standard Test Methods ofAmer AmeryNo ratings yet
- Treatise On Photography On Collodion, 1858 EditionDocument190 pagesTreatise On Photography On Collodion, 1858 EditionJonNo ratings yet
- PiDocument30 pagesPiSai Praneethtej SaspretNo ratings yet
- Shawon Notes Chem A LevelsDocument38 pagesShawon Notes Chem A LevelsEfath UddinNo ratings yet
- Nucleation and GrowthDocument23 pagesNucleation and Growthyinglv67% (3)
- Synthesis of Cesium Octacyanomolybdate (V) - and Cesium Cyanotungstate (V) Dihydrate: A More Successful Method OctaDocument3 pagesSynthesis of Cesium Octacyanomolybdate (V) - and Cesium Cyanotungstate (V) Dihydrate: A More Successful Method OctaDabasish DekaNo ratings yet
- 〈670〉 Auxiliary Packaging ComponentsDocument7 pages〈670〉 Auxiliary Packaging Componentsmehrdarou.qaNo ratings yet
- Asphalt EnesDocument6 pagesAsphalt EnesCathuu CatalinaNo ratings yet
- 38 PDFDocument3 pages38 PDFsanjay ukalkarNo ratings yet
- Wan Noor Afifah BT Wan YusoffDocument33 pagesWan Noor Afifah BT Wan YusoffThilagavathyNo ratings yet
- Tutorial 3Document3 pagesTutorial 3Weixuan SeeNo ratings yet
- Magnetohydrodynamics, An Overview: The Effects of Magnets On FluidsDocument14 pagesMagnetohydrodynamics, An Overview: The Effects of Magnets On FluidsamsteefenNo ratings yet
- Salt AnalysisDocument6 pagesSalt AnalysisARTHUR BALAJI RNo ratings yet
- Qualitative Testfor Elementsin Organic CompoundDocument8 pagesQualitative Testfor Elementsin Organic Compoundidon'tgiveachogiwaNo ratings yet