Professional Documents
Culture Documents
Article - Current Status and Applications of New Embryo Technologies
Uploaded by
Ravindu PereraOriginal Title
Copyright
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentArticle - Current Status and Applications of New Embryo Technologies
Uploaded by
Ravindu PereraCurrent Status and Applications of New
Embryo Technologies in Dairy Herd
Management
Peter J. Hansen
Dept. of Animal Sciences, University of Florida, PO Box 110910, Gainesville, FL 32611-0910
Email: Hansen@animal.ufl.edu
Take Home Message
Superovulation remains the standard for embryo transfer in Canada but
commercial organizations now exist for creation of in vitro produced
embryos
In vitro produced embryos can be used to enhance genetic selection and
to select calves of predetermined sex
In crossbreeding systems, in vitro produced embryos can be used to
maintain heterosis by transferring F1 embryos into F1 recipients
As compared to AI, embryo transfer can improve fertility of lactating cows
when herd fertility is very low (as shown for heat stress) but not when
herd fertility is moderate or high
Current Status of Embryo Transfer in Canada
Embryo transfer has been performed commercially in cattle since the 1970’s.
Since that time, an impressive array of new technologies has been
incorporated into systems for embryo production and transfer. These
technologies have resulted in new methods for producing embryos, for
improving embryo quality, for long-term storage of embryos and oocytes, and
for screening of embryos for important genes.
WCDS Advances in Dairy Technology (2007) Volume 19: 217-226
text.indd 217 2/13/07 9:34:36 AM
218 Hansen
Figure 1. Different schemes for producing embryos for embryo transfer
programs in cattle.
Shown in Figure 1 is a schematic illustration of common methods to generate
embryos for embryo transfer programs in cattle. A fundamental goal of
embryo transfer programs is to increase the number of embryos produced by
one female. The typical way to achieve this increase in embryo numbers is to
cause the cow to ovulate multiple follicles by injecting follicle stimulating
hormone to recruit multiple follicles to grow and ovulate. An alternative
method is to harvest multiple oocytes from a cow and fertilize the oocytes in
vitro. Two methods of harvest exist. Oocytes can easily be removed from
ovaries collected at slaughter. Commercial production of embryos produced
using ovaries recovered at an abattoir is now practiced. In addition, oocytes
can be harvested from the living cow by piercing the vaginal wall with a
needle to allow puncture of follicles on the ovary that have been visualized by
ultrasound. This procedure, called transvaginal, ultrasound-guided oocyte
aspiration (oocyte pickup or OPU for short), allows for oocytes to be obtained
multiple times, including during pregnancy.
The standard for embryo production remains the superovulation procedure
coupled with non-surgical recovery and transfer that was first developed in the
1970s. Reuben Mapletoft from the University of Saskatchewan and Karen
McDermott from the Canadian Embryo Transfer Association maintain records
on the scope of embryo transfer in Canada. For 2005, a total of 46,975
embryos produced by superovulation were transferred. Of these, 39,211 or
83% were transferred to dairy cattle. In contrast, only 142 embryos produced
by in vitro fertilization were recorded as being transferred.
text.indd 218 2/13/07 9:34:37 AM
Current Status and Applications of New Embryo Technologies 219
Given that there were 1.1 million dairy cows in Canada in 2005, it is obvious
that embryo transfer remains a rare event. It is not too surprising that this is
the case. Embryo transfer is expensive and time-consuming and the
advantages over artificial insemination not obvious in many cases. In vitro
production has its own, additional problems. As compared to embryos
produced by superovulation, in vitro produced (IVP) embryos don’t survive
freezing as well, generate lower pregnancy rates following transfer, and can
be associated with larger neonatal death losses.
Most of those roughly 47,000 embryos transferred in Canada in 2005 were
probably used to increase the number of offspring from elite females from
purebred herds. This remains an important use of embryo transfer and
embryo transfer has had a significant impact on breeding strategies for dairy
cattle. As of November 2006, 39 of the 122 Canadian-proven Holstein bulls
actively marketed in Canada were produced by embryo transfer.
One consequence of new technologies being incorporated into embryo
production systems is embryo transfer is being used in new ways for genetic
selection and for other purposes also. The purpose of this paper is to highlight
some new uses of embryo transfer based on production of embryos produced
in vitro.
Large-Scale Production of In Vitro Produced Embryos
Worldwide, the number of cattle embryos produced in vitro has grown rapidly.
Data complied by Michel Thibier and colleagues on behalf of the International
Embryo Transfer Society indicates 265,991 recorded transfers of IVP
embryos in 2005 vs. 612,178 transferred embryos produced by
superovulation. In 2000, by contrast, only 41,761 IVP embryos were
transferred as compared to 528,540 embryos produced by superovulation.
This growth in transfer of IVP embryos has been accompanied by the
development of private and university organizations devoted to the production
of embryos in vitro. Examples of commercial organizations in the United
States include Trans Ova of Sioux Center, Iowa, Evergen Biotechnologies of
Storrs, Connecticut, and Embogen of Gainesville, Florida. An example of a
Canadian company involved in bovine in vitro fertilization is L’Alliance Boviteq
of Saint-Hyacinthe, Québec. Currently, exportation of IVP embryos from the
United States to Canada is not allowed but intergovernmental discussions on
developing a protocol for importing IVP embryos into Canada are planned for
2007.
A simple and inexpensive way to obtain the oocytes needed to produce large
numbers of embryos in vitro is to harvest oocytes from ovaries recovered at
an abattoir. This approach is utilized extensively by companies like Trans Ova
text.indd 219 2/13/07 9:34:38 AM
220 Hansen
and Evergen. There are two disadvantages to such a source of oocyte. The
obvious one is that the donor is usually anonymous and there is little
opportunity for donor selection beyond breed. On average, however, the
oocyte donors are not inferior cows genetically, even though they ended up
sent to slaughter. Jack Rutledge at the University of Wisconsin has estimated
that the predicted merit for milk yield in cows at slaughter is nearly the same
as the average cow. A second disadvantage relates to transmission of
disease. There is no case report of an embryo transmitting an infectious
disease but the possibility, even if remote, cannot be discounted. When
ovaries used to obtain oocytes are obtained without knowledge of the donor, it
is not possible to verify the health status of the donor. Moreover, ovaries and
oocytes from different cows usually come in contact with each other so that
there is a possibility for cross-contamination.
Use of OPU to obtain oocytes for in vitro fertilization does not have the
disadvantages listed above. The identity of the female is ensured so that
selection of elite females can be achieved and the health history of the donor
is known. Also, cows can be subjected to the procedure frequently
(sometimes twice a week) and oocytes can be harvested during pregnancy.
However, OPU is an expensive process that requires trained personnel and
can cost about US$150-200 per procedure in addition to costs for in vitro
fertilization. Reports on the average number of oocytes from unstimulated Bos
taurus cows harvested can vary from 0 to 5 so that one or two embryos per
session would be a reasonable yield. This yield can be increased to about
three embryos per session if cows are treated with follicle stimulating
hormone to increase follicular development. It is likely that superovulation is
superior to OPU in terms of cost-effectiveness in most cases. Exceptions
may include cases where one wants to maximize embryo yield per year
(because OPU can be done as frequently as twice a week) or where an
individual female does not respond well to superovulation.
One problem with the IVP embryo is that it doesn’t survive freezing very well.
Some companies and research groups have reported acceptable pregnancy
rates for cryopreserved in vitro produced embryos but others do fresh
transfers only. Worldwide, 31% of transferred IVP embryos in 2005 were
cryopreserved vs. 46% for embryos produced by superovulation. Transferring
embryos fresh without cryopreservation means that it is necessary to
synchronize production of the embryo with stage of the estrous cycle of the
recipient cow. Fortunately, timed embryo transfer can be performed using
similar hormonal treatments used for timed artificial insemination. An example
of one effective protocol is in Table 1. Using this procedure with lactating cow
recipients during cool weather in Florida, the calving rate for a group of 44
recipients was 23%.
text.indd 220 2/13/07 9:34:38 AM
Current Status and Applications of New Embryo Technologies 221
Table 1. Recommended schedule for timed embryo transfer using in
vitro produced embryos.
Day Activity
Day 0 Inject 100 μg GnRH, i.m.
Day 7 Inject 25 mg prostaglandin F2 , i.m.
Day 9 Inject 100 μg GnRH, i.m.
Day 10 (Anticipated day of ovulation)
Day 17 Transfer fresh in vitro produced embryo fertilized on Day 10
Transfer should be performed only on cows with a corpus
luteum - place embryo in the uterine horn adjacent to the corpus
luteum
Use of In Vitro Produced Embryos for Genetic
Improvement
Genetic selection can be enhanced by increasing the number of offspring per
female. Doing so increases selection intensity (a smaller proportion of
females are selected to produce the next generation) and also the accuracy of
selection (because more records are available from individual animals and
their relatives). The most common way to increase number of offspring per
female is to utilize superovulation or OPU. In addition, some genetic progress
can be obtained using oocytes recovered from ovaries at slaughter.
Following slaughter, ovaries typically end up as part of the offal. Rendering
ovaries represents a waste of genetic resources when the cow from which
those ovaries came is a genetically superior cow. There are some limited
efforts being taken to utilize those genetic resources, but much more could be
done. Bomed Inc. of Madison, Wisconsin performs in vitro fertilization using
oocytes obtained from individual donor cows or from pooled batches of
ovaries from cull cows of contracted dairies. Embryos are transferred without
freezing and are typically transferred to cows from the herd in which the
embryo was derived. There is also one purebred Holstein dairy in California
that has organized its own in-house in vitro fertilization laboratory.
Changes in the way in which dairy cows are identified should make it possible
for large-scale selection of individual donor cows at the abattoir. Theoretically,
an organization producing embryos in vitro could identify individual cows
electronically and query databases to obtain information on herd of origin or
individual performance records. One model for such an organization would be
a farmer cooperative where embryos were returned to the participating herds.
The resources required to build and operate an in vitro fertilization laboratory
are modest.
text.indd 221 2/13/07 9:34:39 AM
222 Hansen
Regardless of the source of oocyte, in vitro fertilization should be performed
using elite sires because the costs of the semen are amortized over several
offspring. With artificial insemination (AI), and an assumed calving rate to a
single insemination of 25%, one would need to inseminate eight cows to
obtain one heifer. Using in vitro fertilization, a single straw could typically be
used to inseminate 100 oocytes or more. If 100 oocytes were fertilized with a
straw of semen, 25 transferable embryos or more could likely be produced. At
a 25% calving rate to embryo transfer with lactating recipients, the single
straw of semen would produce about 3 heifer calves.
Embryo Transfer as a Means of Sex Selection
It has long been possible to sex embryos. This is accomplished by taking a
small biopsy of cells from the embryo and then performing a laboratory
procedure called PCR to determine whether the Y chromosome is present.
Embryo biopsy requires specialized training and is expensive. Now, however,
the advent of sex-sorted semen makes it possible to produce embryos of the
desired sex so that biopsy to identify sex is not needed.
Sex-sorted semen is prepared by using an instrument called a flow cytometer
to separate X-bearing sperm from Y-bearing sperm based on the slightly
larger size (i.e., higher DNA content) of the former. Straws of sex-sorted
sperm are now commercially available. Unfortunately, use of sexed-sorted
sperm in AI is associated with reduced fertility because of sperm damage
during the sexing procedure and because the number of sperm packaged per
straw is reduced. This fact, as well as the high costs, has limited the use of
sex-sorted sperm for artificial insemination under commercial conditions to
non-lactating heifers.
The poor fertility of sex-sorted sperm means that its use is also not
recommended for superovulated donor cattle. It does, however, work with in
vitro fertilization. Calves produced by in vitro fertilization with sex-sorted
sperm where X-bearing sperm are retained are 90% female or greater. In the
United States, there are currently at least two companies selling embryos
produced with sex-sorted sperm – Evergen and Trans Ova. In Canada,
L’Alliance Boviteq recently obtained a license to produce bovine embryos
using sexed semen. Cost will likely vary depending upon source of oocyte
and other considerations. At the time of writing, Trans Ova was selling
Holstein embryos produced with sex-sorted semen and oocytes obtained at
an abattoir for US$135.
Recently, the effectiveness of sex-sorted sperm was demonstrated in a large-
scale study involving exportation of IVP embryos to China (Xu et al., 2006).
Holstein embryos were produced in the United States using in vitro
fertilization with sex-sorted sperm or conventional sperm. Embryos were then
text.indd 222 2/13/07 9:34:40 AM
Current Status and Applications of New Embryo Technologies 223
transferred to either Chinese Native Yellow cattle or Holstein cattle in China.
As a control, some commercially-purchased embryos produced by
superovulation were also transferred. As shown in Table 2, 41% of the IVP
embryos established a pregnancy that was maintained until at least
pregnancy diagnosis at Day 70 of gestation. Pregnancy rates were the same
for embryos produced with sex-sorted sperm and conventional sperm. Of the
458 calves born from the IVP embryos produced using sex-sorted sperm at
the time of publication, 96.5% were female.
Table 2. Effect of embryo production system on pregnancy rates in
Chinese Native Yellow and Holstein recipients receiving cryopreserved
Holstein embryos (From Xu et al., 2006).
Embryo type Number of transfers Pregnancy rate, 70
days after transfer (%)
IVP, vitrified, sex-sorted 3,627 41
sperm
IVP, vitrified, 481 41
conventional sperm
In vivo, superovulation, 192 53
conventional freezing
There has been only one published study in which IVP embryos produced
using sex-sorted sperm have been transferred to lactating dairy cows. In that
study, in Wisconsin (Wilson et al., 2005), pregnancy rates achieved following
transfer of fresh embryos produced by sex-sorted sperm was 19% (n=138) vs.
36% for AI using conventional semen (n=526). There was no group in which
embryos were produced with conventional sperm so it was not determined
whether the lower pregnancy rates for embryo transfer was due to use of sex-
sorted sperm.
Embryo Transfer in Crossbreeding Systems
Crossbreeding has been put forward as a method for improving fertility and
health traits of dairy cattle and for reducing the inbreeding present in all major
North American dairy breeds. One limitation of crossbreeding is the loss of
heterosis and increased variation that occurs for the offspring of F1 females.
In a two-breed rotational breeding system, the percent heterosis falls from
100% in the F1 generation to 50% in the F2 and 75% in the F3 before
stabilizing at 63-69% in subsequent generations. Loss of heterosis can be
prevented in large part by using a three or four breed rotational breeding
system involving unrelated breeds although variation in type may make
management more difficult.
text.indd 223 2/13/07 9:34:41 AM
224 Hansen
Another method to prevent loss of heterosis is to maintain the F1 generation
indefinitely using embryo transfer. Embryos can be produced in vitro or in vivo
through appropriate matings (for example use Swedish Red semen and
Holstein oocytes to produce F1 embryos for a herd of Swedish Red x Holstein
cows). With such a scheme, both the F1 female and her offspring experience
100% heterosis.
Can Embryo Transfer Be Used to Improve Fertility?
Conception rates in lactating cows are much lower than for non-lactating
heifers and have been on the decline for 30 years of more. Theoretically,
embryo transfer should improve fertility in the lactating cow because
pregnancy failure brought about by defects in the oocyte, ovulation,
fertilization, or early embryonic development can be bypassed. Typically,
embryo transfer is performed at Day 7 after estrus and only those embryos
that have successfully completed development to that point are transferred.
Whether or not embryo transfer can improve fertility in lactating cows at
current levels of technology is not certain. Only a very few embryo transfer
studies have been performed with lactating cows. An illustration of the
effectiveness of embryo transfer for improving fertility of lactating cows is
provided in Figure 2. These data come from an experiment by Rodriques et
al. (2004) in Brazil using lactating Holstein cows. Pregnancy rates were
greater for embryo transfer recipients than inseminated cows in the summer,
when fertility in the inseminated group was compromised by heat stress. In
the winter, when fertility in the inseminated group was high, there was no
improvement in pregnancy rate caused by embryo transfer.
text.indd 224 2/13/07 9:34:41 AM
Current Status and Applications of New Embryo Technologies 225
Figure 2. Differences in pregnancy rate between embryo transfer
recipients and inseminated animals in lactating cows in Brazil. Lactating
Holstein cows were either inseminated or received a fresh or frozen-thawed
embryo produced by superovulation. Data are either the number
pregnant/number inseminated or number pregnant/number receiving an
embryo. Months in which the average ambient air temperature was less than
22.5oC are shown with the gray bar. Note that embryo transfer increased
pregnancy rate in hot weather but not in cool weather. Data are redrawn from
Rodrigues et al. (2004).
The benefits of embryo transfer for lactating cows during heat stress have
been shown several times for herds in Florida and Brazil. Perhaps embryo
transfer will also improve fertility in herds with low fertility for reasons other
than heat stress. This hasn’t yet been proven.
In a recent study with lactating cows in Wisconsin (Sartori et al., 2006) live
calving rate was 26% (47 cows pregnant of 184 cows total) for cows receiving
an embryo produced by superovulation vs. 28% (56 cows pregnant of 203
cows total) for inseminated cows. It is likely that further improvements in the
process for production, selection, and transfer of embryos as well as in
recipient management will be needed before embryo transfer can be an
effective and economical method to improve fertility in herds with reasonable
fertility.
text.indd 225 2/13/07 9:34:42 AM
226 Hansen
Acknowledgements
The author thanks the following people for providing information about current
commercial uses of embryo transfer: Fuliang Du (Evergen Biotechnologies),
Lorraine Leibfried-Rutledge (Bomed), and Chris Sigurdson (Trans Ova).
References
Rodrigues, C.A., H. Ayres, E.L. Reis, M. Nichi, G.A. Bo, and P.S. Baruselli
(2004). Artificial insemination and embryo transfer pregnancy rates in
high production Holstein breedings under tropical conditions. Proc. 15th
Congr. Anim. Reprod. 2: 396 (abstr.).
Sartori, R., A. Gumen, J.N. Guenther, A.H. Souza, D.Z. Caraviello, and M.C.
Wiltbank (2006) Comparison of artificial insemination versus embryo
transfer in lactating dairy cows. Theriogenology 65:1311-1321.
Xu, J., Z. Guo, L. Su, T.L. Nedambale, J. Zhang, J. Schenk, J.F. Moreno, A.
Dinnyes, W. Ji, X.C. Tian, X. Yang, and F. Du (2006) Developmental
potential of vitrified Holstein cattle embryos fertilized in vitro with sex-
sorted sperm. J. Dairy Sci. 89:2510-2518.
Wilson, R.D., K.A. Weigel, P.M. Fricke, J.J. Rutledge, M.L. Leibfried-
Rutledge, D.L. Matthews, and V.R. Schutzkus (2005) In vitro
production of Holstein embryos using sex-sorted sperm and oocytes
from selected cull cows. J. Dairy Sci. 88:776-782.
text.indd 226 2/19/07 11:37:30 AM
You might also like
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- 1 - Overview of The Aquaculture Feed IndustryDocument19 pages1 - Overview of The Aquaculture Feed IndustryRavindu PereraNo ratings yet
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Chapter 14Document30 pagesChapter 14Ravindu PereraNo ratings yet
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- ICT EnglishDocument14 pagesICT EnglishRavindu PereraNo ratings yet
- Chapter 8Document15 pagesChapter 8Ravindu PereraNo ratings yet
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (400)
- Chapter 6Document36 pagesChapter 6Ravindu PereraNo ratings yet
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- Chapter 13Document20 pagesChapter 13Ravindu PereraNo ratings yet
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Chapter 9Document18 pagesChapter 9Ravindu PereraNo ratings yet
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Chapter 2Document35 pagesChapter 2Ravindu PereraNo ratings yet
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- Chapter 5Document30 pagesChapter 5Ravindu PereraNo ratings yet
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Chapter 3Document34 pagesChapter 3Ravindu PereraNo ratings yet
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Evaluation of Chick Quality : Which Method Do You Choose?Document4 pagesEvaluation of Chick Quality : Which Method Do You Choose?Ravindu PereraNo ratings yet
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Chapter 4Document33 pagesChapter 4Ravindu PereraNo ratings yet
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- ManihotDocument201 pagesManihotRavindu PereraNo ratings yet
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- Ñksidf.A Iudfhdackh Yd Iuiaó S L%SHDJ, SHDocument9 pagesÑksidf.A Iudfhdackh Yd Iuiaó S L%SHDJ, SHRavindu PereraNo ratings yet
- Sustainable Soil ManagementDocument2 pagesSustainable Soil ManagementRavindu PereraNo ratings yet
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Stingless Bee KeepingDocument13 pagesStingless Bee KeepingRavindu PereraNo ratings yet
- Sri Lanka Nes 4 3 WebDocument104 pagesSri Lanka Nes 4 3 WebRavindu PereraNo ratings yet
- Recruitment and SelectionDocument11 pagesRecruitment and SelectionRavindu PereraNo ratings yet
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (345)
- Sample Teaching Guide Catchup Health Grade4Document7 pagesSample Teaching Guide Catchup Health Grade4Hazel JusosNo ratings yet
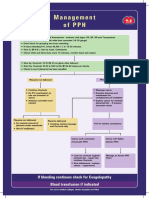
- Management of PPHDocument1 pageManagement of PPH098 U.KARTHIK SARAVANA KANTHNo ratings yet
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- Note: Refer To The Structures Above and Familiarize The Functions of Each PartsDocument2 pagesNote: Refer To The Structures Above and Familiarize The Functions of Each PartsRam August100% (1)
- Karryn's Prison Titles and Passives.Document32 pagesKarryn's Prison Titles and Passives.Virgo SeptianNo ratings yet
- Livrogustavo78 3 330 74332 8Document94 pagesLivrogustavo78 3 330 74332 8claudiiNo ratings yet
- Physiological DevelopmentDocument26 pagesPhysiological DevelopmentMaraNo ratings yet
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- Newborn Appraisal BenitaDocument172 pagesNewborn Appraisal BenitaBenudavid David100% (2)
- When Does Human Life Begin?: Starter: Record YourDocument12 pagesWhen Does Human Life Begin?: Starter: Record Yourapi-375411758100% (2)
- Vagina:: Female Reproductive System (Human), Details ..Document8 pagesVagina:: Female Reproductive System (Human), Details ..Giovanni CeneriniNo ratings yet
- Health Law - 9th SemDocument5 pagesHealth Law - 9th SemsoumyaNo ratings yet
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (121)
- Waiters Uterine Curette PDFDocument1 pageWaiters Uterine Curette PDFmp1757No ratings yet
- It's Perfectly NormalDocument4 pagesIt's Perfectly NormalEric Fuseboxx50% (4)
- 16 Simple Yoga Asanas To Increase Fertility in WomenDocument24 pages16 Simple Yoga Asanas To Increase Fertility in WomenSuganya K. SelvakumarNo ratings yet
- Abnormal Uterine ActionDocument14 pagesAbnormal Uterine ActionSwara RamtekeNo ratings yet
- Corpul Galben: - Glanda Endocrina TemporaraDocument6 pagesCorpul Galben: - Glanda Endocrina TemporaraIoana GrayNo ratings yet
- Hilaria Sas 8Document3 pagesHilaria Sas 8Christy Mae Batucan HilariaNo ratings yet
- Female Reproductive SystemDocument7 pagesFemale Reproductive SystemRem M RollinasNo ratings yet
- Pemasangan Kontrasepsi Implan Dan Alat Kontrasepsi Dalam Rahim Di Dusun XVIII Kecamatan Percut Sei TuanDocument9 pagesPemasangan Kontrasepsi Implan Dan Alat Kontrasepsi Dalam Rahim Di Dusun XVIII Kecamatan Percut Sei TuanPuskesmas CaritaNo ratings yet
- 2018521625078-Mohan EthirajanDocument7 pages2018521625078-Mohan EthirajanMohan EthirajanNo ratings yet
- Reproduction in Plants and Animals WorksheetDocument11 pagesReproduction in Plants and Animals Worksheetkarishma mannarNo ratings yet
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- Effects of Educational Intervention On Sexual Health Knowledge, Attitude and Behavioral Practices: A School-Based Study in Calabar, NigeriaDocument12 pagesEffects of Educational Intervention On Sexual Health Knowledge, Attitude and Behavioral Practices: A School-Based Study in Calabar, NigeriaIJAR JOURNALNo ratings yet
- Congenital GenitourinaryDocument93 pagesCongenital GenitourinaryEllen AngelNo ratings yet
- Data Obat Lengkap-SIAP IMPORTDocument9 pagesData Obat Lengkap-SIAP IMPORTRegita Ragil SejahteraNo ratings yet
- Office of The Sangguniang Kabataan: Local Youth Development Plan (Lydp)Document17 pagesOffice of The Sangguniang Kabataan: Local Youth Development Plan (Lydp)Sandy Andrea Gabas75% (4)
- Hottest FamilyDocument92 pagesHottest Familymalik asadNo ratings yet
- Abortion Is The Practice of Terminating A Pregnancy Resulting In, or CloselyDocument2 pagesAbortion Is The Practice of Terminating A Pregnancy Resulting In, or CloselyAlejandra BenjumeaNo ratings yet
- Consti OnlineclassDocument18 pagesConsti OnlineclassJesa FormaranNo ratings yet
- The CW Jane The Virgin 1x01 PilotDocument60 pagesThe CW Jane The Virgin 1x01 PilotTan TanNo ratings yet
- Chapter 5 RetnoDocument4 pagesChapter 5 RetnoRetno AWNo ratings yet
- Maternal & Child Health Nursing Flashcards QuizletDocument1 pageMaternal & Child Health Nursing Flashcards QuizletJosh PagnamitanNo ratings yet