Professional Documents
Culture Documents
Quantity Changes of Reactants / Products Total Time of Reaction
Uploaded by
Alia KhanOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Quantity Changes of Reactants / Products Total Time of Reaction
Uploaded by
Alia KhanCopyright:
Available Formats
17. What are the factors that affect the rate of reaction?
Temperature
Pressure
Concentration
Surface area
Catalyst
18. What is effect of temperature on the rate of reaction?
When we raised a temp. the molecules bounce around a lot more. They have more energy.
When they bounce around more, they are more likely to collide. When the molecules are slower
and collide less, the temp. drop lowers the rate of reaction.
19. What is effect of concentration on the rate of reaction?
If there is more of a substance in a system,there is greater choice that molecules will collide,
If there is less of something in a system,there will be fewer and reaction will probably happens at
slower rate.
20. What is effect of pressure on the rate of reaction?
Pressure affects reaction rate when you look at gases especially. The gas particles become close
together increasing the frequency of collisions. This means particles are more likely to react.
21. What is effect of catalyst on the rate of reaction?
A catalyst is a substance which speeds up chemical reaction but is chemically unchanged as its
end. When the reaction has finished, the mass of catalyst is same at beginning.
There are two types of catalysts:
1. Positive catalyst
2. Negative catalyst
22. Why catalyst so important for industry?
Products can be made more quickly saving time and money.
Catalyst reduce the need for high temperature, saving fuel and reducing pollutants.
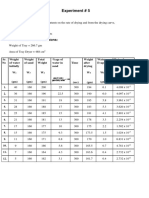
23. How can we find the average rate of reaction?
Reaction rate is the measure how fast a reaction occurs, or how much the reactant/product
change in a period of time.
Quantity changes of reactants / products
Total time of Reaction
You might also like
- Contemporary Catalysis: Fundamentals and Current ApplicationsFrom EverandContemporary Catalysis: Fundamentals and Current ApplicationsNo ratings yet
- Chemical Kinetics Project of Class 12thDocument16 pagesChemical Kinetics Project of Class 12thDhairya Tamori74% (58)
- Chapter No 2 Process Selection: 2.1 Manufacturing Processes For Caustic SodaDocument28 pagesChapter No 2 Process Selection: 2.1 Manufacturing Processes For Caustic SodaAlia Khan100% (1)
- Chemical Change 1Document36 pagesChemical Change 1CheloGraceTiozonAmparadoNo ratings yet
- Rates of ReactionsDocument55 pagesRates of ReactionsJOSHUA NYANGENANo ratings yet
- Factors Affecting The Rate of ReactionDocument3 pagesFactors Affecting The Rate of Reactionapi-234891239100% (1)
- Reaction Rate NOTES HandoutDocument1 pageReaction Rate NOTES HandoutHorizon 99No ratings yet
- Physical Scie.Document2 pagesPhysical Scie.Khate G. PrestozaNo ratings yet
- Unit - The Periodic TableDocument20 pagesUnit - The Periodic TableMaurya AdeshraNo ratings yet
- Ate of Hemical Eactions: by StudentDocument17 pagesAte of Hemical Eactions: by StudentDyah Ayu pramoda wardaniNo ratings yet
- Factors Affecting The Rate of ReactionDocument2 pagesFactors Affecting The Rate of ReactionMr GaMeR SMNo ratings yet
- CHEMISTRYDocument10 pagesCHEMISTRYlifep7417No ratings yet
- Definitions - Topic 7 Chemical Reactions - CAIE Chemistry IGCSE PDFDocument2 pagesDefinitions - Topic 7 Chemical Reactions - CAIE Chemistry IGCSE PDFAtif BakhshNo ratings yet
- Speed of Reaction SummaryDocument3 pagesSpeed of Reaction Summarychong5660% (5)
- 2.2.4 Rate of Chemical ReactionDocument12 pages2.2.4 Rate of Chemical Reactiondansochristiana574No ratings yet
- Short Note Chemistry Form 5-Chapter 1 Rate of ReactionDocument4 pagesShort Note Chemistry Form 5-Chapter 1 Rate of Reactionsalamah_sabri100% (1)
- Physci Simple Collision TheoryDocument34 pagesPhysci Simple Collision TheoryLovely benzelNo ratings yet
- Rate of ReactionDocument12 pagesRate of ReactionRobin CabralNo ratings yet
- Chemical Kinetics & Equilibrium: Froilan Aron S. Faraon, R.PHDocument34 pagesChemical Kinetics & Equilibrium: Froilan Aron S. Faraon, R.PHKenneth TrogonNo ratings yet
- Collision Theory & CatalystDocument33 pagesCollision Theory & CatalystSHEENA MAE DALGUNTASNo ratings yet
- 1126pm - 47.epra Journals 5023Document3 pages1126pm - 47.epra Journals 5023No NameNo ratings yet
- Physical Change: Chemical ReactionsDocument9 pagesPhysical Change: Chemical ReactionsAishi GuptaNo ratings yet
- For Exer 3Document16 pagesFor Exer 3Louiegi AlvarezNo ratings yet
- Pointers To Review in General Chemistry 2Document3 pagesPointers To Review in General Chemistry 2jkarllucasNo ratings yet
- Factors Affecting Reaction RatesDocument9 pagesFactors Affecting Reaction Ratesscsa31619No ratings yet
- C8 Rates of ReactionDocument25 pagesC8 Rates of Reactionshayaanzaman0No ratings yet
- Speed of Reactions PDFDocument8 pagesSpeed of Reactions PDFafoo1234No ratings yet
- Chem 3Document103 pagesChem 3César ArenasNo ratings yet
- The Rate of Chemical Reaction - NotesDocument6 pagesThe Rate of Chemical Reaction - Notesshawaiz.m.malik2010No ratings yet
- Rates of ReactionDocument3 pagesRates of ReactionTofunmi OyeboluNo ratings yet
- Concentration of ReactantsDocument4 pagesConcentration of ReactantsFernalyn Elaine TabilogNo ratings yet
- Factors Affecting Rate of ReactionDocument3 pagesFactors Affecting Rate of ReactionKyle BantaNo ratings yet
- Factors Affecting Rate of ReactionDocument6 pagesFactors Affecting Rate of Reactionjohnrey_lidres100% (4)
- Rate of Reaction XI MIPA 8Document19 pagesRate of Reaction XI MIPA 8Shofwa AnnisaNo ratings yet
- Factors That Affect Reaction RatesDocument4 pagesFactors That Affect Reaction RatesenieynazNo ratings yet
- Chemical Reaction IDocument2 pagesChemical Reaction IPrinceblesed EdemNo ratings yet
- Information and Communication Technology in Chemistry: Title: Collision Theory (Simulation)Document10 pagesInformation and Communication Technology in Chemistry: Title: Collision Theory (Simulation)z890% (1)
- Rate of ReactionDocument23 pagesRate of ReactionVirly vcNo ratings yet
- Chemical RactionsDocument21 pagesChemical RactionsJamaika D. BIERNEZA100% (1)
- Rates of ReactionDocument6 pagesRates of ReactionRednaxela OnalaNo ratings yet
- Chemical Kinetics: Submitted To: Submitted byDocument17 pagesChemical Kinetics: Submitted To: Submitted byTarun Pratap Singh50% (2)
- Measuring Rates of Reaction: Things To MeasureDocument3 pagesMeasuring Rates of Reaction: Things To MeasureMuhammad AfnanNo ratings yet
- Chemical Kinetics Project of Class 12thDocument16 pagesChemical Kinetics Project of Class 12thSubham PrajapatNo ratings yet
- The Collision TheoryDocument5 pagesThe Collision TheoryRhea PardiñasNo ratings yet
- AssignmentDocument5 pagesAssignmentAnsel MercadejasNo ratings yet
- Chemical Kinetics: Factors Affecting Reaction RateDocument4 pagesChemical Kinetics: Factors Affecting Reaction RateMontassar DridiNo ratings yet
- Reviewer in ChemDocument1 pageReviewer in ChemAlaine Chloe Bondoc AlvaroNo ratings yet
- Rate of Chemical ReactionDocument3 pagesRate of Chemical Reactionbehatimonzanto0No ratings yet
- Part 5 Reaction Dynamics NotesDocument10 pagesPart 5 Reaction Dynamics NotesKeira WhitfordNo ratings yet
- 1a Rates of Reaction Slides PDFDocument26 pages1a Rates of Reaction Slides PDFArshad KhanNo ratings yet
- Jawahar Navodaya Vidyalaya, Purulia (W. B) : Name-Smriti Mandal Class-Xii Aissce Roll No.Document19 pagesJawahar Navodaya Vidyalaya, Purulia (W. B) : Name-Smriti Mandal Class-Xii Aissce Roll No.smritymandal883No ratings yet
- Lesson 6 Collision Theory and Chemical Reaction RateDocument33 pagesLesson 6 Collision Theory and Chemical Reaction Ratealliah nibayNo ratings yet
- Chemical KineticsDocument31 pagesChemical KineticsAnonymous LnQ4lBXiPjNo ratings yet
- Reaction Rate NotesDocument10 pagesReaction Rate NotesvinaybharadwajbsNo ratings yet
- Chemistry Unit 8Document3 pagesChemistry Unit 8cindyNo ratings yet
- 377chemistry Unit 4 Notes CompleteDocument65 pages377chemistry Unit 4 Notes Completemuddasser91100% (3)
- 5 Rate of Chemical ReactionDocument20 pages5 Rate of Chemical ReactionjoshuaandreicsabellanoNo ratings yet
- Gen - Chem 2 Q3 Module6 7Document16 pagesGen - Chem 2 Q3 Module6 7Kenneth HernandezNo ratings yet
- ChemistryDocument49 pagesChemistryAnam FNo ratings yet
- Practice Makes Perfect in Chemistry: Kinetics and Equilibrium with AnswersFrom EverandPractice Makes Perfect in Chemistry: Kinetics and Equilibrium with AnswersNo ratings yet
- HEC Pakistan - Recommendations - 28 - JULY - 2021Document160 pagesHEC Pakistan - Recommendations - 28 - JULY - 2021Alia KhanNo ratings yet
- List of Documents To Be Attached With The Application Form For Registration As Professional Engineer (Pe) (Through Epe)Document6 pagesList of Documents To Be Attached With The Application Form For Registration As Professional Engineer (Pe) (Through Epe)Alia KhanNo ratings yet
- Annex 6: Content and Format of Iee and Siee: Environment Assessment & ReviewDocument8 pagesAnnex 6: Content and Format of Iee and Siee: Environment Assessment & ReviewAlia KhanNo ratings yet
- Production of 250 MTPD Sodium HydroxideDocument99 pagesProduction of 250 MTPD Sodium HydroxideAlia KhanNo ratings yet
- Connect: Picnic at Sunway LagoonDocument28 pagesConnect: Picnic at Sunway LagoonAlia KhanNo ratings yet
- DHA Flyer 2020Document2 pagesDHA Flyer 2020Alia KhanNo ratings yet
- Production of 250 MTPD Sodium HydroxideDocument18 pagesProduction of 250 MTPD Sodium HydroxideAlia KhanNo ratings yet
- Group 1: SR - No# Group Number Roll Number Names CGPA Proposed TopicDocument3 pagesGroup 1: SR - No# Group Number Roll Number Names CGPA Proposed TopicAlia KhanNo ratings yet
- Introduction of Production of Caustic SodaDocument14 pagesIntroduction of Production of Caustic SodaAlia Khan100% (2)
- The Purpose of Sulfur Guard BedDocument1 pageThe Purpose of Sulfur Guard BedAlia Khan100% (1)
- Project Title: Production of Sodium Hydroxide From Brine Electrolysis Using Membrane CellDocument2 pagesProject Title: Production of Sodium Hydroxide From Brine Electrolysis Using Membrane CellAlia KhanNo ratings yet
- Fauji Fertilizer Company Limited Management Trainees Test (Schedule 2021)Document1 pageFauji Fertilizer Company Limited Management Trainees Test (Schedule 2021)Alia KhanNo ratings yet
- Block Diagram of DHDS: Feed Tank Coalescer Filter Heat Exchanger FurnaceDocument2 pagesBlock Diagram of DHDS: Feed Tank Coalescer Filter Heat Exchanger FurnaceAlia KhanNo ratings yet
- Experiment # 5: Objective of The StudyDocument2 pagesExperiment # 5: Objective of The StudyAlia KhanNo ratings yet
- Industrial Accidents: - Safety and Loss PreventionDocument13 pagesIndustrial Accidents: - Safety and Loss PreventionAlia KhanNo ratings yet
- Bzu, Bahadur Campus, Layyah: Award List (Viva Voce)Document3 pagesBzu, Bahadur Campus, Layyah: Award List (Viva Voce)Alia KhanNo ratings yet
- Experiment No. 2: ObjectDocument3 pagesExperiment No. 2: ObjectAlia KhanNo ratings yet
- 1038-Fourth Merit List-BBA (Morning) 2020Document3 pages1038-Fourth Merit List-BBA (Morning) 2020Alia KhanNo ratings yet