Professional Documents
Culture Documents
Mechanisms of Thiamin Deficiency in Chronic Alcoholism1: Anastacio M. Hoyumpa, JR., M.D
Uploaded by
Rubie Ann TillorOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Mechanisms of Thiamin Deficiency in Chronic Alcoholism1: Anastacio M. Hoyumpa, JR., M.D
Uploaded by
Rubie Ann TillorCopyright:
Available Formats
Mechanisms of thiamin deficiency in chronic
alcoholism1 -3
Anastacio M. Hoyumpa, Jr., M.D.
ABSTRACT In the United States and other developed countries thiamin deficiency is often
related to chronic alcoholism. A number of mechanisms may be involved in the pathogenesis of
thiamin deficiency in the alcoholic population. An important cause is inadequate intake of thiamin.
Moreover, there may be decreased converstion of thiamin to the active coenzyme, reduced hepatic
storage of the vitamin in patients with fatty metamorphosis, ethanol inhibition of intestinal thiamin
transport, and impaired thiamin absorption secondary to other states of nutritional deficiency. The
present discussion focuses on the mechanism of ethanol-related thiamin malabsorption. Under
normal conditions thiamin transport in animals and humans is biphasic. At low or physiological
thiamin concentrations, transport is a saturable, carrier-mediated, active process; but at higher
concentrations, the transport of thiamin is predominantly passive. Ethanol reduces the rate of
intestinal absorption and the net transmural flux of thiamin. Furthermore, ethanol inhibits only
the active and not the passive component of thiamin transport by impeding the cellular exit of
thiamin across the basolateral or serosal membrane. The impairment of thiamin movement out of
the enterocyte correlates with a fall in the activity of Na-K ATPase. Bound to the basolateral
membrane, Na-K ATPase is believed to be involved in the kinetics of active transport. Ethanol
also increases the fluidity of enterocyte brush border and basolateral membranes. Since ethanol
increases membrane fluidity it is possible that the impairment of thiamin transport and the
diminution of Na-K ATPase activity may be related, at least partly, to a physical perturbation of
the enterocyte membrane. Am. J. Clin. Nutr. 33: 2750-2761, 1980.
Thiamin was the first member of the vi- include insufficient intake of the vitamin,
tamin B complex to be chemically identified. decreased formation of thiamine pyrophos-
In the presence of ATP it is converted to phate, reduced hepatic thiamin storage, in-
thiamin pyrophosphate which functions in hibition of intestinal thiamin transport by
carbohydrate metabolism as a cocnzyme in ethanol, and secondary impairment of thia-
the decarboxylation of pyruvic and a-keto- mm absorption due to ethanol-related nutri-
glutaric acids and in the utilization of pcntose tional deficiencies. Each of these factors will
in the hexose monophosphate shunt. A seri- be discussed separately, but emphasis will be
ous deficiency of thiamin leads to accumula- placed on the effect of ethanol on intestinal
tion of pyruvate and a-kctoglutarate in the transport of thiamin as this has been the
blood and a decrease in the activity of trans- subject of active investigation.
ketolasc. Clinically, lack of thiamin is char-
acterized by neurological and cardiovascular Insufficient thiamin intake
disturbances that give risc to Wernicke-Kor-
sakoff syndrome, peripheral neuritis, and The requirement of thiamin depends upon
beri-beri heart disease. the metabolic rate. In man the minimum
In underdeveloped countries thiamin defi- requirement is 0.33 mg/l000 cal but to pro-
cicncy is generally the result of poor dietary
practices such as eating mainly polished rice From
I the Veterans Administration Medical Center
and other food preparations containing anti- and Vanderbilt University School of Medicine, Nash-
thiamin factors, but in the United States and vile, TN.
2Supported by the Medical Research Service of the
other developed countries thiamin deficiency
Veterans Administration.
is often related to chronic alcoholism (1-4). 3Address reprint requests to: Anastacio M. Hoyumpa,
The circumstances which may be responsible Jr., M.D., VA Medical Center, Nashville, Tennessee
for this association are listed in Table 1 and 37203.
2750 The American JournalofClinical Nutrition 33: DECEMBER1980, pp. 2750-2761. Printed in U.S.A.
Downloaded from https://academic.oup.com/ajcn/article-abstract/33/12/2750/4692383
by University of Glasgow user
on 04 April 2018
THIAMIN DEFICIENCY IN CHRONIC ALCOHOLISM 2751
TABLE 1 was measured in rats fed ethanol chronically
Possible mechanisms of thiamin deficiency in chronic
there was a suggestive decrease (13) which,
alcoholism
however, did not reach statistical significance
1. Inadequate thiamin intake possibly because of the small number of de-
2. Decreased activation ofthiamin to thiamin pyrophos- terminations. Despite these suggestive find-
phate
ings the abnormal conversion of thiamin to
3. Reduced hepatic storage of thiamin
4. Inhibition of intestinal thiamin transport
the active coenzyme remains to be proved.
5. Impairment of thiamin absorption due to ethanol- Studies must be designed to rule out impaired
related nutritional deficiency states intestinal absorption of thiamin as a major
factor contributing to the lowered concentra-
tions of hepatic thiamin or decreased activity
vide a margin of safety a daily consumption of thiamin-dependent enzymes.
of 0.5 mg/l000 cal is recommended by the
Food and Nutrition Board of the National
Research Council. The actual daily consump- Reduced hepatic storage
tion of thiamin by healthy subjects usually
The liver is a principal storage depot for
ranges from 0.4 to 2.0 mg (5). In contrast,
thiamin, and normally contains 2.0 to 7.6
alcoholic subjects tend to consume less than
mg/g moist tissue (14) compared to 1.4 to 4.1
0.29 mg/l000 kcal of thiamin (2). This find-
in the brain and 2.8 to 7.9 in the heart. In
ing is consistent with the observation that of
patients with severe alcoholic liver disease,
3000 alcoholic patients, admitted to a large the thiamin content may be reduced by as
municipal hospital because of withdrawal much as 73% (1, 15). Inhibition of thiamin
symptoms or intercurrent illness, evidence of uptake into isolated rat hepatocytes by
dietary deficiency occurred during periodic ethanol was demonstrated in one study (16),
binges of alcoholic drinking in 40%. Pro- but not in another (17). Moreover, in liver
longed dietary deficiency alternating with a perfusion studies, ethanol was shown to cause
marginal or normal diet during periods of the release of thiamin from the liver (18).
abstinence was observed in 25%, and contin- These factors, along with the decrease in
uous dietary deficiency was a feature in an- functioning hepatic parenchyma in the pres-
other 35% (6). In such patients the presence ence ofsevere fatty metamorphosis, may con-
of anorexia and intake of inadequate and tribute to decreased storage of thiamin.
unbalanced diet during alcohol consumption
(which provide only “empty calories”) con-
tribute to the dietary deficiency. Ethanol inhibition of thiamin absorption
Characteristics of normal thiamin transport
Decreased formation of thiamin
pyrophosphate Many studies of intestinal thiamine trans-
port have been carried out in the past utilizing
The conversion of thiamin to thiamin py- different methods and various animal species,
rophosphate may be impaired in the presence often with conflicting results. From an exten-
of alcoholic liver injury, possibly because of sive review of the literature Rindi and Ven-
decreased availability or use of ATP (6) and tura (19) concluded that the intestinal trans-
may be associated with decreased activity of port of thiamin can be accomplished by both
pyruvate decarboxylase and transketolase active and passive processes. This was con-
due to an apoenzyme (7) or magnesium (8, 9) firmed in studies in rats in which intestinal
deficiency. The decrease in hepatic and cere- transport ofthiamin was shown to be biphasic
bral transketolase activity is accompanied by both by in vivo and in vitro methods (20). At
a parallel fall in thiamin concentrations in low or physiologic thiamin concentrations
the liver (10, 1 1) and brain (10). Furthermore, (< 1 .0 /LM), transport was a saturable, carrier-
since the transketolase activity correlates with mediated process requiring energy (Fig. 1 and
thiamin pyrophosphate concentrations (12), 2) while at higher or pharmacological thiamin
these findings suggest a lowering in the con- concentrations, transport was predominantly
centrations of thiamine pyrophosphate. In- by a passive, nonsaturable process (Fig. 3). It
deed, when hepatic thiamin pyrophosphate has also been shown that the rate of thiamin
Downloaded from https://academic.oup.com/ajcn/article-abstract/33/12/2750/4692383
by University of Glasgow user
on 04 April 2018
2752 HOYUMPA
NET FLUX STUDIES
TRANSPORT AGAINST CONCENTRATION GRADIENT
S/M
RATIO
O.2pM 2OjjM
TH IAMIN CONCENTRATION
EFFECT OF VARIOUS INHIBITORS
O.2pM 2OpM
NORMAL
PT DNP NEM OUA PT DNP OUA
PT-PYRITHIAMIN DNP-DINITROPHENOL NEMN-ETHYLMALEIMIDE OUAOUABAIN
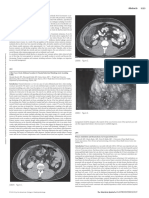
FIG. 1. Transmural net flux studies, in vitro, using evertedjejunal sacs incubated at 37 C with Kreb’s bicarbonate
buffer pH 7.4. Identical concentrations of ‘4C-thiamin hydrochloride were placed in the mucosal and serosal
compartments. Movement against a concentration gradient was deemed present if at the end of I hr incubation, the
concentration of labelled thiamin was greater in the serosal fluid, so that the serosal/mucosal concentration (S/M)
ratio was greater than the original 1.0. The top panel shows that 0.2 tM thiamin (open bar) was transported against
a concentration gradient (S/M ratio 1.5) while 20 sM thiamin (shaded bar) was not (S/M ratio, 1.0). The lower panels
compare the effect of various inhibitors on the net transmural flux of 0.2 eM thiamin (left) and 20 .tM thiamin (right).
These compounds inhibited thiamin in low, but not in high, concentration. Reproduced with permission (20).
transport is greater in the proximal than in centrations (20). Second, to traverse the lipid
the distal intestinal segment (Fig. 4). cell membrane, a water-soluble substance like
A tentative scheme of thiamin transport is thiamin requires special transport mecha-
shown in Figure 5. It can be seen that as nisms such as provided by mobile carriers. A
thiamin moves from the mucosal to the se- thiamin binding protein has been isolated
rosal compartment it encounters certain phys- and characterized in microorganisms (22-24),
icochemical barriers, and a number of events, but such a carrier protein has not yet been
some of which are still poorly defined, take identified in mammalian tissue. However, the
place. First, thiamin must get across the un- presence of such a carrier in rats is suggested
stirred water layer (21) adjacent to the cell by the observed saturation phenomenon at
membrane. Stirring of this water layer de- low thiamin concentrations (Fig. 2). Further-
creases its thickness, lowers the Km and fa- more, the competitive inhibition of thiamin
cilitates the transfer of thiamin in low con- transport exhibited by pyrithiamin (Fig. 2)
Downloaded from https://academic.oup.com/ajcn/article-abstract/33/12/2750/4692383
by University of Glasgow user
on 04 April 2018
THIAMIN DEFICIENCY IN CHRONIC ALCOHOLISM 2753
z 250 NO PYRITHIAMIN
!!
c’J before subtracting passive component
200
I 50
I 00
01 0.2 03 04 05 0 20
THIAMIN CONCENTRATION (jiM)
FIG. 2. Unidirectional jejunal uptake of thiamin in vitro. Jejunal uptake rates in the absence of pyrithiamin
show saturation
(#{149}-#{149}) kinetics. The broken line (0- - -0) indicates uptake after subtracting the passive component,
calculated as the product of the permeability coefficient and the thiamin concentration. The addition of pyrithiamin,
2 zM (X-X) abolished the saturability. Each value represents the mean of 10 to 20 sacs. Reproduced with
permission (20).
5--NORMAL CONTROL (Nli) active, energy-requiring process which ena-
)O---x PYRITHIAMIN (N-7)
-
ADDED TO
bles the transfer of thiamin against a concen-
z INCUBATION MEDIA tration and electrochemical gradient (20).
Fourth, cyclic AMP has been shown to en-
N
hance transport of sodium and of certain
I- p > 05
amino acids (26), but it did not influence
‘1>- intestinal thiamin transport in rats (A. M.
Hoyumpa, Jr., and S. G. Nichols, unpub-
lished observations). Fifth, Na-K ATPase
(0_
2 which is located mainly in the basolateral
0-’
membrane (27) is believed to play an impor-
U
-J
0 tant role in active transport (28) by providing
0 energy through the hydrolyses of ATP. In
z
support of the role of Na-K ATPase in thia-
2
mm transport are the observations that va-
0 10 20 30 40 50
sopressin treatment in chicks increases intes-
THIAMIN CONCENTRTI0N (pM) tinal Na-K ATPasc activity and thiamin
transport (29) and that decreasing Na-K
FIG. 3.
Unidirectional jejunal uptake of high con-
centrations of thiamin, in vitro. Normal jejunal uptake
ATPasc activity by ethanol or ouabain cx-
(#{149}-#{149})
shows a linear relationship to thiamin concentra- posure is associated with a fall in thiamin
tions in the mucosal compartment. Addition of pyrithia- transport from the cell to the serosal com-
mm (X- - -X) causes no significant change in thiamin partment (see below).
uptake. Reproduced with permission (20).
In contrast to these characteristics of low
thiamin concentrations, the movement of
and other structural analogs (25) would sug- high concentrations ofthiamin is not affected
gest that these analogs vie with thiamin for by the thickness of the unstirred water layer,
common binding sites. Third, the adverse the presence of a structural analog, anoxia,
effect of anoxia, low temperature, and the hypothermia, or metabolic inhibitors. The
metabolic inhibitors dinitrophenol and N- part played by paracellular pathways in the
cthylmaleimide on the transport of low con- transport of thiamin across the intestine has
centrations of thiamin (Fig. 1) suggest an not been studied.
Downloaded from https://academic.oup.com/ajcn/article-abstract/33/12/2750/4692383
by University of Glasgow user
on 04 April 2018
2754 HOYUMPA
TISSUE UPTAKE SEROSAL APPEARANCE
I-
z
0< 05 I-.-
a)
JEJUNUM 0”
0.:
o
0
aE
Ui0
0
-)
2 4
MINUTES MINUTES
FIG. 4. Comparison of thiamin tissue uptake (left panel) and rate of serosal appearance (right panel) between
jejunum and ileum in normal rats.
Thiamin
low concentration
high concentration
FIG. 5. Tentative scheme of intestinal thiamin transport. The different bamers to the transport ofthiamin in low
concentrations are represented. Thiamin crosses the unstirred water layer adjacent to the cell membrane and its
subsequent movement across the brush border membrane itself may involve a carrier protein. Analogs like
pyrithiamin compete with thiamin for binding sites on this carrier. Once inside the enterocyte, thiamin appears to be
phosphorylated, but is subsequently dephosphorylated as it leaves the cell. However, the role of phosphorylation in
thiamin transport is not completely settled. Dinitrophenol may inhibit mitochondrial oxidative phosphorylation; N-
ethylmaleimide may interfere with Na-K ATPase activity. Like ouabain, ethanol may impede thiamin transport
across the basolateral membrane by also inhibiting Na-K ATPase. In high concentrations, thiamin moves through
the enterocyte by simple diffusion and is not affected by these factors.
A number of studies have been carried out pounds in the form of tri-, di- (thiamin py-
to determine the role of phosphorylation in rophosphate) and monophosphate. The phos-
relation to thiamin transport. Thiamin pre- phorylated esters form as much as 60-80% of
sented at the mucosal side accumulates in the thiamin in the intestinal tissue. Subsequently
intestinal cell (30-34) as phosphorylated com- thiamin is dephosphorylated (35) and only
Downloaded from https://academic.oup.com/ajcn/article-abstract/33/12/2750/4692383
by University of Glasgow user
on 04 April 2018
THIAMIN DEFICIENCY IN CHRONIC ALCOHOLISM 2755
free thiamin can be detected in the serosal unpublished observations). Thus, the dual
compartment by in vitro studies (36). This system of intestinal thiamin transport noted
may not be the case when circulation is intact, in rats was observed to apply also to humans.
since in vivo studies indicate that 57% of
Effect of ethanol
thiamin recovered in the portal blood is phos-
phorylated (34). Although thiamin undergoes Impairment of intestinal absorption of
phosphorylation in the cell, a number of ob- thiamin has been well-documented in a sub-
servations suggest that this process may not stantial number of chronic alcoholic patients
be critical for thiamin transport. First, phos- (47, 48). To understand the underlying mech-
phorylation of thiamin is not influenced by anism(s) of thiamin malabsorption in alco-
sodium lack, whereas thiamin transport is holism, the acute and chronic effects of
dependent on sodium (20, 34, 37, 38). Second, ethanol on thiamin transport, on Na-K ATP-
thiamin uptake proceeds at a decreasing rate ase activity, and on membrane fluidity were
along the intestinal tract, with the rate of studied in rats.
uptake highest in the duodenum and lowest Acute effect. The acute intragastric admin-
in the ileum, while the distribution of thiamin istration of ethanol, 50 to 750 mg/100 g body
pyrophosphokinase (the enzyme responsible weight, to rats reduced the absorption of low,
for phosphorylation) along the digestive tract but not of high, concentrations of thiamin
does not parallel the rate of thiamin transport (49). Once attained, this effect of ethanol was
(34). Third, the activity of thiamin pyrophos- not made worse by a further increase in the
phokinase is localized mainly in the soluble ethanol dose, and it was reversible as ethanol
cell fraction and scarcely detected in the disappeared from the blood stream. More-
brush border membrane which is the princi- over, it was unrelated to changes in osmolality
pal site of thiamin uptake. Fourth, thiamin or to any structural alterations of the intes-
transport may be dissociated from phospho- tinal mucosa. In vitro studies also showed
rylation in the isolated hcpatocyte ( 17). Fi- that ethanol inhibited the net transmural flux
nally, mutant strains of Escherichia coli which of thiamin in low, but not in high, concentra-
arc incapable ofphosphorylating free thiamin tions (Fig. 6). Subsequent in vitro studies
nevertheless maintain their ability to trans- localized the ethanol site of action. The up-
port thiamin (39). take of thiamin into the intestinal epithelial
Although the information gathered from cells was unimpeded by ethanol, but the
studies in animals and microorganisms pro- movement of low concentrations of thiamin
vide some useful insight into thiamin trans- (0.2 and 0.5 /iM) from the cells to the serosal
port in general, their relevance and applica- compartment was significantly impaired (Fig.
bility to man require confirmation. In the 7A). In contrast, the transport of high con-
past, in vivo studies in man have failed to centrations of thiamin (20 and 50 jiM) was not
define the precise kinetics of intestinal trans- affected (Fig. 7B), in keeping with the dual
port of thiamin (40-45). We have recently system ofthiamin transport. It was also noted
studied the uptake of thiamin by the small that ouabain, a known inhibitor of Na-K
intestinal mucosa obtained during endoscopic ATPase, exerted a similar effect on thiamin
examination. The results (A. M. Hoyumpa, transport with respect to its cellular uptake
Jr., R. Strickland, J. Schechan, G. Yarbor- and exit; this finding suggested that the im-
ough, and S. Nichols, unpublished data) in- pairment of thiamin exit from the cell may
dicate that at low thiamin concentrations (< be related to ethanol inhibition of Na-K
2.0 LM), uptake is a saturable phenomenon ATPase activity in the basolateral membrane.
and is diminished by pyrithiamin, anoxia and Effect of acute ethanol exposure on Na-K
sodium lack. Phosphorylation and accumu- A TPase. To determine the effect of ethanol
lation ofthiamin (0.2 tM) in intestinal mucosa directly on Na-K ATPase activity, brush bor-
were also observed by Rindi and Ferrari (46) dcr and basolatcral membranes were isolated
in human surgical specimens. In contrast, at from rat enterocytes and Na-K ATPase activ-
higher thiamin concentration (5 to 50 LM) ity was measured (27). Na-K ATPase activity
intestinal mucosal uptake exhibits first order was noted to reside mainly in the basolateral
kinetics (A. M. Hoyumpa, Jr., R. Strickland, membranes. In vitro exposure of the basolat-
J. Sheehan, G. Yarborough, and S. Nichols, eral membrane to 0.5 M ethanol reduced Na-
Downloaded from https://academic.oup.com/ajcn/article-abstract/33/12/2750/4692383
by University of Glasgow user
on 04 April 2018
2756 HOYUMPA
K ATPase activity by at least 50% (Fig. 8) ethanol was administered by gavagc one hour
and exposure to a range of ethanol concen- before the isolation of the membranes and
trations (0. 1 to 1.0 M) produced a dose-depen- measurement of Na-K ATPase (26). Further-
dent but nonlinear inhibition (not shown). more, the fall in Na-K ATPase activity was
This inhibitory action was reproduced when accompanied by a decrease in the rate of
thiamin transport from the cell interior to the
serosal compartment (Fig. 9). Thus, it is cvi-
CONTROL ALCOH0L
RATIO
dent that ethanol inhibits both Na-K ATPase
20
activity and serosal transport (exit) of small
quantities of thiamin. However, whether this
association is causal or casual remains to be
established. It is also pertinent to examine in
the future whether ethanol interferes with
thiamin phosphorylation and dephophoryla-
tion mechanisms.
Effect of chronic ethanol ingestion. The rel-
evance of the above observations, obtained
with single doses of ethanol given to normal
rats, to the pathogenesis ofthiamin deficiency
O2pM 2OpM
in chronic alcoholism in man is uncertain.
THIAMIN CONCENTRATION
Therefore, the influence of chronic ethanol
FIG. 6. Effect ofalcohol (ethanol) on net transmural
administration on thiamin transport was
flux ofthiamin in vitro. Ethanol 2.5% was placed in both
mucosal and serosal compartments. As shown in the left studied. Rats were fed an ethanol-containing
panel, ethanol inhibited the movement of thiamin in low liquid diet as described by Dc Carli and
concentration (0.2 LM) so that the serosal/mucosal con- Lieber (50) for 6 to 8 weeks. Thiamin trans-
centration ratio was reduced from the control value of
port was measured using everted intestinal
I .5 (open bar) to I .0 (hatched bar). In contrast, as shown
segments and correlated with Na-K ATPase
in the right panel, ethanol had no effect in the net flux of
thiamin in high concentration (20 riM). Reproduced with activity in the basolateral membrane (13).
permission (49). Results indicated that chronic ethanol feed-
B. HIGH THIAMIN CONCENTRATION
300C
LI CONTROL
- 2S000ALCOHOL
. p>O5
3 2000
500
‘ p>.O5
000
I ,
I- 500
II JL.
O.2jiM O.5pM 2OpM 5OpM
THIAMIN CONCENTRATION THIAMIN CONCENTRATION
FIG. 7. Effect of alcohol (ethanol) on the transport of thiamin from the intestinal epithelium to the serosal
compartment (serosal exit). Low thiamin concentrations (0.2 and 0.5 tM) are shown in the left, high concentrations
(20 and 50 /LM) on the right. Alcohol inhibited the transport of low, but not of high, thiamin concentrations.
Reproduced with permission (49).
Downloaded from https://academic.oup.com/ajcn/article-abstract/33/12/2750/4692383
by University of Glasgow user
on 04 April 2018
THIAMIN DEFICIENCY IN CHRONIC ALCOHOLISM 2757
ing impaired neither thiamin uptake nor se- ethanol concentrations in the plasma and
rosal exit when the ethanol concentrations in intestinal lumen were raised to 185 and 318
the plasma and intestinal lumen were 40 and mg/ 100 ml by an additional single intragas-
39 mg/lOO ml, respectively. Similarly, Na-K tric dose of ethanol, 250 mg/lOO g body
ATPase activity in the basolateral membrane weight, the exit of thiamin (0.2 and 0.5 /.LM)
was unaffected (Fig. 10). However, when the across the serosal membrane was significantly
decreased along with a corresponding reduc-
tion ofthe basolateral membrane Na-K ATP-
asc activity (Fig. 11). These data suggest that
the inhibition of thiamin transport is depen-
>-c
dent more on the systemic ethanol concentra-
>a tions present rather than on the duration of
p- 0
exposure to ethanol and that thiamin mal-
absorption in this model may be intermittent.
That thiamin malabsorption may result from
chronic ethanol ingestion was also shown by
others (5 1). Any intermittent malabsorption
of thiamin would clearly become more sig-
nificant if associated with marginal thiamin
intake as is often the case with alcoholic
JEJUNUM ILEUM patients.
FIG. 8. Effect of ethanol 0.5 H on basolateral mem- Ethanol and membranefluidity. An optimal
brane Na-K ATPase activity. fluidity or microviscosity of the cell mcm-
IS
-J
0
IO z
I
U’
% 5
-J
a.
‘a
I.--.
-J
0
>- 2
I-.
I
U
t.2100 z
U
a...
i-c
_J
-J
z- U
I-
z
ETHANOL DOSE
(mg/bOG B.W.)
FIG. 9. Effect of acute ethanol ingestion on thiamin transport across the basolateral membrane to the serosal
compartment and on the basolateral membrane Na-K ATPase activity. The study shows a correlation between the
impairment ofthiamin transport (A) and the fall in Na-K ATPase activity (B) I hr after the intragastric administration
of ethanol 50 to 750 mg/lOO g. The ethanol concentrations in plasma (C) and intestinal lumen (D) are also shown.
Reproduced with permission (27).
Downloaded from https://academic.oup.com/ajcn/article-abstract/33/12/2750/4692383
by University of Glasgow user
on 04 April 2018
.-.PAIR-FED CONTROL
2758 HOYUMPA
A THIAMIN TRANSPORT B. Na-K ATPase
- 2C
UJE o--0CHRONIC ETHANOL
ZN-)
a:
0QJ
Q_ 0
(flU ,
Z
a: - IC
OE
ZcCo
- UJO
w;::
O(f)
,Alrr p>O5
? (r’.9-ISporsl
OL)
I I I
Oa
0.2 0.4 0.6 0.8
THIMIN CONCENTRATIONS FED ETHANOL
(jM) CONTROL
FIG. 10. Lack ofeffect ofethanol feeding for 6 to 8 weeks on thiamin transport across the serosal membrane (A)
and on the basolateral membrane Na-K ATPase activity (B). Ethanol concentrations in the plasma and intestinal
lumen were low (see text). Reproduced with permission (13).
A THIAMIN TRANSPORT B. Na-K ATPase
C 2C >-
:: PAIR-FED CONTROL >-
Zr))
I-a c: ETHANOL
U
a a 150
0 p<.O25
Q-LU
.C_J
‘
I
a:Z IC 100
p<.OOI
ZUO
..(D 5 50
100 22
OL) oE
0c - - - UL.L_O
0.21jM 0.5pM JEJUNAL BASOLATERAL
THIAMIN CONCENTRATION MEMBRANES
FIG . 1 1 . Effect of chronic ethanol feeding (6 to 8 weeks) plus an acute dose of intragastric ethanol, 250 mg/l00
g, on thiamin transport across the serosal membrane (left) and on basolateral membrane Na-K ATPase activity
(right). Reproduced with permission (13).
brane is required for normal cell functions. der and basolateral membranes in a dose-
Since Na-K ATPase is a membrane protein, dependent manner, similar to the data ob-
it is possible that the decrease in its activity tamed in mouse erythrocytcs and brain mem-
may be related to changes in its microenvi- branes (53). In contrast, membranes obtained
ronment. In addition, physical perturbation from rats fed ethanol (Dc Carli-Lieber diet)
of the membrane bilayer may so alter the for 12 weeks did not alter membrane fluidity,
disposition of transport channels as to inter- a finding also noted in the erythrocyte of
fere with the transport process. Therefore, the mice exposed to ethanol for 8 days (54).
physical state of the cell membrane was as- However, the acute addition of ethanol in
sessed by determining membrane fluidity by vitro to membranes from rats on the chronic
electron paramagnetic resonance (52). The ethanol diet produced a dose-dependent in-
spin label used, N-oxyl 4’, 4’-dimethyloxazo- crease in the fluidity of brush border and
lidine derivative of 5 ketostearic acid, moni- basolateral membranes (52). These fluidity
tors the region of the polar heads close to the data obtained by different groups of investi-
water-lipid interface. Both the enterocyte gators echo the findings on thiamin transport
brush border and basolateral membranes and Na-K ATPase activity with respect to the
were examined. Acute in vitro exposure to effect ofacute and chronic ethanol treatment.
ethanol, 0. 1 to 1 .0 M, increased the fluidity Moreover, it is believed that chronic ethanol
(decreased microviscosity) of the brush bor- exposure leads to membrane adaptation. This
Downloaded from https://academic.oup.com/ajcn/article-abstract/33/12/2750/4692383
by University of Glasgow user
on 04 April 2018
THIAMIN DEFICIENCY IN CHRONIC ALCOHOLISM 2759
may explain why no abnormality in trans- in the pathogenesis of thiamin deficiency in
port, ATPase activity, and fluidity was de- chronic alcoholism. It is also likely that sev-
tectcd. The adaptive change may be charac- eral of these mechanisms may be involved
terized by an increase in the cholesterol con- concurrently in an individual patient, al-
tent on the membrane and a reduction in the though it may be difficult to determine the
proportion of polyunsaturated fatty acids exact contribution of each factor.
(55). These modifications in the lipid com-
position of the membrane tend to increase References
membrane viscosity and to minimize the flui-
1. LEEVY, C. M., H. BAKER, W. TEN HovE, 0. FRANK
dizing influence ofcthanol. Although the per- AND G. R. CHERRICK. B-complex vitamins in liver
turbation in membrane fluidity brought disease of the alcoholic. Am. J. Clin. Nutr. 16: 339,
about by ethanol may be nonspecific, it may 1965.
account, at least partly, for the ethanol-re- 2. NEVILLE, J. N., J. A. EAGLES, G. SAMSON AND R. E.
OLsoN. Nutritional status ofalcoholics. Am. J. Clin.
lated changes in membrane permeability, en-
Nutr. 21: 1329, 1968.
zyme activity or intestinal transport, includ- 3. WooD, B., K. J. BREEN AND D. G. PENINGTON.
ing that of thiamin. Thiamine status in alcoholism. Australian New Zea-
land Med. 7: 475, 1977.
Nutritional deficiency and thiamin transport 4. HELL, D., AND P. Six. Thiamine-, riboflavin- und
pyridoxin-versorgung bei chronischen alkoholismus.
Dtsch. Med. Wochschr. 102: 962, 1977.
In addition to the direct effect of ethanol
5. WOODHILL, J. M., AND S. NOBILE. Thiamine in the
on thiamin transport, it is conceivable that 1970 Australian diet with special reference to cereals
specific states ofnutritional deficiency related and the assessment of thiamine status. Internat. J.
to chronic ethanol consumption may interfere Vitamin Nutr. Res. 42: 435, 1972.
with thiamin transport secondarily. For in- 6. LEEVY, C. M., AND H. BAKER, Vitamins and alco-
holism. Am. J. Clin. Nutr. 21: 1325, 1968.
stance, folate deficiency which commonly oc-
7. FENNELLY, J., 0. FRANK, H. BAKER AND C. M.
curs in chronic alcoholism may be associated LEEVY. Red blood cell transketolase activity in mal-
with decreased thiamin content in the blood nourished alcoholics with cirrhosis. Am. J. Clin.
and liver in rats, suggesting impaired absorp- Nutr. 20: 946, 1967.
8. ZIEVE, L., W. M. DOIZAKI AND L. E. STENROOS.
tion of thiamin (56). Indeed, folate deficient
Effect of magnesium deficiency on growth response
rats absorbed thiamin less efficiently than to thiamine of thiamine-deficient rats. J. Lab. Clin.
pair fed controls (57). Folate repletion re- Med. 72: 261, 1968.
versed the thiamin malabsorption. Although 9. ZIEVE, L., W. M. DOIZAKI AND L. E. STENROOS.
minor histological changes were noted in the Effect of magnesium deficiency on blood and liver
transketolase activity and on the recovery of enzyme
jejunum (but not in the ileum), these were
activity in thiamine-deficient rats receiving thiamine.
not deemed sufficient to account for the de- J. Lab. Clin. Med. 72: 268, 1968.
crease in thiamin absorption, since only the 10. ABE, T., AND Y. ITOKAWA. Effect ofethanol admin-
low and not the high concentration of thiamin istration on thiamine metabolism and transketolase
activity in rats. Internat. J. Vitamin Nutr. Res. 47:
was affected. These data imply that folate, in
307, 1977.
some as yet undcfmcd ways, plays a role in 1 1. FRANK, 0., AND H. BAKER. Vitamin profile in rats
maintaining the integrity of the active trans- fed stock or liquid ethanolic diets. Am. J. Clin. Nutr.
port process of thiamin. In rats a deficiency 33: 221, 1980.
in vitamin B6 or B12 may also lead to de- 12. WARNOCK, L. G., C. R. PRUDHOMME AND C. WAG-
NER. The determination of thiamin pyrophosphate
creased tissue stores of thiamine (58). In ad-
in blood and other tissues and its correlation with
dition, thiamin deficiency itself may alter the erythrocyte transketolase activity. J. Nutr. 108: 421,
kinetics of thiamin transport (59). As a result 1978.
of reduced unstirred water thickness, the Km 13. HOYUMPA, A. M., S. NicHoLs, G. I. HENDERSON
AND S. SCHENKER. Intestinal thiamin transport: Ef-
of thiamin is reversibly decreased, so that
fect ofchronic ethanol administration in rats. Am. J.
thiamin untake at very low concentrations is Clin. Nuts. 31: 938, 1978.
increased, probably as a compensatory mcch- 14. LAMDEN, M. P. Thiamine. V. Occurrence in foods.
anism. The influence of protein or caloric In: The Vitamins, (2nd ed), edited by W. H. Sebrell
deprivation or both on thiamin transport has and R. S. Harris. New York: Academic Press, p. 116,
1972, vol.V.
not been determined.
15. FRANK, 0., A. LUISADA-OPPER, M. F. SORRELL, A.
From the foregoing discussion, it is appar- D. THOMSON AND H. BAKER. Vitamin deficits in
ent that several mechanisms may be involved severe alcohol fatty liver of man calculated from
Downloaded from https://academic.oup.com/ajcn/article-abstract/33/12/2750/4692383
by University of Glasgow user
on 04 April 2018
2760 HOYUMPA
multiple reference points. Exptl. Molec. Biol. 15: port of thiamine from rat small intestine. J. Nutr.
191, 1971. Sd. Vitaminol. 20: 163, 1974.
16. CHEN, C-P. Active transport of thiamine by freshly 35. FERRARI, G., G. RINDI AND G. D’ANDREA. The
isolated rat hepatocytes. J. Nutr. Sci. Vitaminol. 24: action of inorganic phosphate on thiamin transport
351, 1978. by rat everted jejunal sacs. Pflugers Arch. 376: 47,
17. LUMENG, L., J. W. EDMONDSON, S. SCHENKER AND 1978.
T. K. Li. Transport and metabolism of thiamin in 36. VENTURA, U., G. ScI0RELLI AND M. V. BONANNI.
isolated rat hepatocytes. J. Biol. Chem. 254: 7265, Transporto della tiamina 4 nell’ intestino de ratto,
1979. in vitro. III. Effetto di inhibitori della fosfatasi intes-
18. SORRELL, M. F., H. BAKER, A. J. BARAK AND 0. tinali sul transporto e sulla fosforilazione della tia-
FRANK. Release by ethanol of vitamins into rat liver mina. Boll Soc. Ital. Sper. 42: 1979, 1966.
perfusates. Am. J. Clin. Nutr. 27: 743, 1974. 37. K0MAI, T., AND H. SHINDO. Metabolic rate and
19. RINDI, G., AND U. VENTURA. Thiamine intestinal mechanism of action of chloroethyl thiamine. III.
transport. Physiol. Rev. 52: 821, 1972. Active transport of thiamine from chick intestine
20. HOYUMPA, A. M., H. M. MIDDLETON, F. A. WILSoN and competitive inhibition by chloroethylthiamine.
and S. SCHENKER. Thiamine transport across the rat J. Vitaminol. 18: 55, 1972.
intestine. Gastroenterology 68: 1218, 1975. 38. FERRARI, G., U. VENTURA AND 0. RIND0. The Na+
21. DIETSCHY, J. M., V. L. SALLEE AND F. A. WILSoN. dependence of thiamine intestinal transport in vitro.
Unstirred water layers and absorption across the Life Sci. 10: 67, 1971.
intestinal mucosa. Gastroenterology 6 1 : 932, 197 1. 39. KAWASAKI, T., AND K. YAMADA. Free thiamine
22. NISHIMUNE, T., AND R. HAYASHI. Thiamine binding transport system in Escherichia coli: evidence for the
and thiamine uptake by escherichia coli. Biochim. existence of an energy-dependent exit process. In:
Biophys. Acta 244: 573, 1971. Thiamine, edited by C. J. Gubler, M. Fujiwara and
23. MATSUURA, A., A. IWASHIMA AND Y. NOSE. Purifi- P. D. Dreyfuss. New York: John Wiley and Sons,
cation of thiamine binding protein from escherichia 1976, p. 83.
coli by affinity chromatography. Biochem. Biophys. 40. ALEXANDER, B., G. LANDWEHR AND F. MITCHELL.
Res. Commun. 51: 241, 1973. Studies ofthiamine metabolism in man. II. Thiamine
24. HENDERSON, G. B., AND E. M. ZEVELY. Binding and and pyrimidine excretion with special reference to
transport of thiamine by lactobacillus casei. J. Bac- the relationship between injected and excreted thia-
teriol. 133: 1190, 1978. mine in normal and abnormal subjects. J. Clin.
25. SHINDO, H., AND T. KOMAI. Metabolic fate and Invest. 25: 294, 1946.
mechanism of action of chloroethylthiamine. I. Ab- 41. FRIEDMAN, T. E., T. C. KMIECIAK, P. A. KEEGAN
sorption of chloroethylthiamine from chick intestine AND B. B. SHEFT. The absorption destruction and
and its competition with thiamine. J. Vitaminol. 18: excretion of orally administered thiamine by human
41, 1972. subjects. Gastroenterology 1 1: 100, 1948.
26. KINZIE, J. L., J. A. FERRENDELLI AND D. H. ALPERS. 42. CAMPBELL, J. A., AND A. B. MoRRIsoN. Some factors
Adenosine cyclic 3’-5’-monophosphate mediated affecting the absorption of vitamins. Am. J. Clin.
transport of neutral and dibasic amino acids in je- Nutr. 12: 162, 1963.
junal mucosa. J. Biol. Chem. 248, 7018, 1973. 43. THOMSON, A. D., AND C. M. LEEVY. Observation on
27. HOYUMPA, A. M., S. G. NICHOLS, F. A. WILsoN AND the mechanism of thiamine hydrochloride absorp-
S. SCHENKER. Effect of ethanol on intestinal (Na,K) tion in man. Clin. Sci. 43: 153, 1972.
ATPase and intestinal thiamine transport in rats. J. 44. THOMSON, A. D., 0. FRANK, H. BAKER AND C. M.
Lab. Clin. Med. 90: 1086, 1977. LEEVY. Thiamine propyldisulfide: Absorption and
28. SKou, J. C. Enzymatic aspects ofactive linked trans- utilization. Ann. Internal Med. 74: 529, 1971.
port of Na+ and K+ through the cell membrane. 45. DESMOND, P. V., C. LOURENZS AND K. J. BREEN.
Prog. Biophys. Molec. Biol. 14: 133, 1964. Thiamine hydrochloride absorption in man: Normal
29. LAZAROV, I. Vitamin B, reabsorption VII. Vasopres- kinetics and absence of acute effect of ethanol. Aus-
sin and thiamine reabsorption. Animal Sci. 13: 95, tralian New Zealand J. Med. 6: 264, 1976.
1976. 46. RINDI, G., AND G. FERRARI. Thiamine transport by
30. RINDI, G., AND U. VENTURA. Phosphorylation and human intestine in vitro. Experientia 33: 21 1, 1977.
uphill intestinal transport of thiamine, in vitro. Ex- 47. TOMASULO, P. A., R. M. KATER AND F. L. IBER.
perientia 23: 175, 1967. Impairment of thiamine absorpition in alcoholism.
31. VENTURA, U., G. FERRARI, R. TAGLIBUE AND G. Am. J. Clin. Nutr. 21: 1340, 1968.
RINDI. A kinetic study of thiamine intestinal trans- 48. THOMSON, A. D., H. BAKER AND C. M. LEEVY.
port in vitro. Life Sci. 8: 699, 1967. Patterns of 355-thiamine hydrochloride absorption
32. FERRARI, G., G. ScI0RELLI, P. DEL P00Gb, U. in the malnourished alcoholic patient. J. Lab. Clin.
VENTURA AND G. RINDI. Free thiamine as the likely Med. 76: 34, 1970.
precursor of endocellular thiamine. Phosphate in 49. HOYUMPA, A. M., K. J. BREEN, S. SCHENKER AND F.
everted rings ofratjejunum. Pflugers Arch. 356: 111, A. WILSON. Thiamine transport across the rat intes-
1975. tine. II. Effect ofethanol. J. Lab. Clin. Med. 86: 803,
33. CUSARO, G., G. RINDI AND G. SCIORELLI. Subcel- 1975.
lular distribution of thiamine pyrophosphokinase 50. DE CARLI, L. M., AND C. S. LIEBER. Fatty liver in
and thiamine-pyrophosphatase activities in rat iso- the rat after prolonged intake of ethanol with a
lated enterocytes. Internat. J. Vitamin Nutr. Res. 47: nutritionally adequate new liquid diet. J. Nutr. 91:
99, 1977. 33, 1967.
34. KOMAI, T., K. KAWAII AND H. SHINDO. Active trans- 5 1. BALAGHI, M., AND R. A. NEAL. Effect of chronic
Downloaded from https://academic.oup.com/ajcn/article-abstract/33/12/2750/4692383
by University of Glasgow user
on 04 April 2018
THIAMIN DEFICIENCY IN CHRONIC ALCOHOLISM 2761
ethanol administration on thiamin metabolism in the tolerance and dependence. Alcoholism: Cliii. ExpI.
rat. J. Nutr. 107: 2144, 1977. Res. 3: 50, 1979.
52. GRAY, J. P., A. M. HOYUMPA, G. D. DUNN, G. I. 56. THOMSON, A. D., D. FRANK, B. DE ANGELES AND
HENDERSON, F. A. WILSON AND L. L. SWIFT. Effect H. BAKER. Thiamine depletion induced by folate
of ethanol (E) in fluidity of enterocyte membranes. deficiency in rats. Nutr. Rep. Internat. 6: 107, 1972.
Clin. Res. 27: 683A, 1979. 57. HOWARD, L., C. WAGNER AND S. SCHENKER. Mal-
53. CHIN, J. H., AND D. B. GOLDSTEIN. Drug tolerance absorption of thiamine in folate deficient rats. J.
in biomembranes. A spin-label study of the effects Nutr. 104: 1024, 1974.
of ethanol. Science 196: 684, 1976. 58. NISHIN0, K., AND Y. IToic,WA. Thiamine metabo-
54. CHIN, J. H., L. M. PARSON AND D. B. GOLDSTEIN. lism in vitamin B6 or vitamin B12 deficient rats. J.
Increased cholesterol content of erythrocyte and Nutr. 107: 775, 1977.
brain membranes in ethanol-tolerant mice. Biochim. 59. HOYUMPA, A. M., S. NIcHoi.s, S. SCHENKER AND F.
Biophys. Acta 513: 358, 1978. A. WILSoN. Thiamine transport in thiamine-defi-
55. LITELETON, J. M., G. R. JOHN AND S. V. GRIEVE. cient rats. Role ofthe unstirred water layer. Biochim.
Alterations in phospholipid composition in ethanol Biophys. Acta 436: 438, 1976.
Downloaded from https://academic.oup.com/ajcn/article-abstract/33/12/2750/4692383
by University of Glasgow user
on 04 April 2018
You might also like
- Psychiatry Student Guide: MEDD 421 Clinical Skills 2019-2020Document25 pagesPsychiatry Student Guide: MEDD 421 Clinical Skills 2019-2020Rubie Ann TillorNo ratings yet
- 110 Genetic Disease in HumansDocument5 pages110 Genetic Disease in HumansQuang Huy PhạmNo ratings yet
- Name and Classification of Drug Mechanism of Action Adverse Effects Nursing Responsibility Keto-Analogues + EAA Trade NameDocument2 pagesName and Classification of Drug Mechanism of Action Adverse Effects Nursing Responsibility Keto-Analogues + EAA Trade NameKrizha Angela NicolasNo ratings yet
- Pharma Lecture With Dr. Maria Yña Eluisia T. Pereyra-Borlongan 1Document13 pagesPharma Lecture With Dr. Maria Yña Eluisia T. Pereyra-Borlongan 1Sammy GirlNo ratings yet
- Drug StudyDocument10 pagesDrug StudyRubie Ann TillorNo ratings yet
- Incorrectly: CorrectlyDocument25 pagesIncorrectly: CorrectlypikachuNo ratings yet
- Science Abc4203Document13 pagesScience Abc4203tb7dcbxfwnNo ratings yet
- 1 43Document6 pages1 43Marko Sanja StikovicNo ratings yet
- Pineal GlandDocument2 pagesPineal GlandYolisNo ratings yet
- Melatonin, Receptors, Mechanism, and Uses: Review ArticleDocument14 pagesMelatonin, Receptors, Mechanism, and Uses: Review ArticleMohsin NazirNo ratings yet
- Coagulation DisordersDocument9 pagesCoagulation DisordersIS99057No ratings yet
- Shrimp: In: Use of Sodium Metabisulfite, AlternativesDocument3 pagesShrimp: In: Use of Sodium Metabisulfite, Alternativesvalerie rosalind angkawidjajaNo ratings yet
- Amino Vita DSDocument1 pageAmino Vita DSMika MinsalanNo ratings yet
- Deficiencia de Tiamina en Perros y GatosDocument8 pagesDeficiencia de Tiamina en Perros y GatosKarleyn Erin Martínez TorresNo ratings yet
- Amino Acid CombiDocument22 pagesAmino Acid CombiJoyce Hanniel CastinoNo ratings yet
- Amine Transporters: Juan Zhen, Maarten EA ReithDocument8 pagesAmine Transporters: Juan Zhen, Maarten EA ReithMurat OzNo ratings yet
- Pineal GlandDocument16 pagesPineal GlandYolisNo ratings yet
- Melamine Toxicity and The Kidney: Special ArticleDocument6 pagesMelamine Toxicity and The Kidney: Special ArticlejeriNo ratings yet
- Tryptophan Metabolism, From Nutrition To Potential Therapeutic Applications - 2011 - Le Floc'h, Otten, MerlotDocument11 pagesTryptophan Metabolism, From Nutrition To Potential Therapeutic Applications - 2011 - Le Floc'h, Otten, MerlotArnulf BultmannNo ratings yet
- Hyper Tyrosine MiaDocument49 pagesHyper Tyrosine Miathaismartins023No ratings yet
- Cases Week 2Document2 pagesCases Week 2JiyahnBayNo ratings yet
- Genetic Defects of Thiamine Transport and MetabolismDocument17 pagesGenetic Defects of Thiamine Transport and MetabolismAndre MattarNo ratings yet
- Bcaromtrans-Protein MetabolismDocument8 pagesBcaromtrans-Protein MetabolismRay Emmanuel Enriquez DomingoNo ratings yet
- Biochem Essential and Non EssentialsDocument2 pagesBiochem Essential and Non EssentialsAlvin Cris RongavillaNo ratings yet
- Vii Urine Screening For Metabolic Disorders PDFDocument4 pagesVii Urine Screening For Metabolic Disorders PDFMariel AbatayoNo ratings yet
- 12 Vit B1Document27 pages12 Vit B1Maya DasmaselaNo ratings yet
- DrugsDocument22 pagesDrugsPAUL MICHAEL G. BAGUHINNo ratings yet
- Homocystinuria, Also Known As Cystathionine Beta Synthase Deficiency or CBS DeficiencyDocument4 pagesHomocystinuria, Also Known As Cystathionine Beta Synthase Deficiency or CBS DeficiencyARyiam GtpNo ratings yet
- Proteins and Protein MetabolismDocument9 pagesProteins and Protein MetabolismclaireNo ratings yet
- Enzymes and BiochemistryDocument4 pagesEnzymes and Biochemistryapi-709276885No ratings yet
- Lipidosis HepaticaDocument45 pagesLipidosis HepaticaJojoa E WilNo ratings yet
- Kompilasi Vit Part 1 Rev1Document84 pagesKompilasi Vit Part 1 Rev1smileyesthiNo ratings yet
- Poster Presentation ICPEBDocument1 pagePoster Presentation ICPEBMonica DrestiaNo ratings yet
- PhenylalanineDocument4 pagesPhenylalaninemaithili hedaooNo ratings yet
- EMTy FAT Falla Endotelial y CV 2018Document2 pagesEMTy FAT Falla Endotelial y CV 2018Nicolás PardoNo ratings yet
- EtymologyDocument3 pagesEtymologyJayrelle D. SafranNo ratings yet
- AntidoteDocument3 pagesAntidotedeanelaylayNo ratings yet
- Feline Hepatic LipidosisDocument46 pagesFeline Hepatic LipidosisAndre Suarez FarfanNo ratings yet
- MOLECULARBIO LESSON 3 Proteins and Mutation EditedDocument13 pagesMOLECULARBIO LESSON 3 Proteins and Mutation EditedLea Chariza PagauisanNo ratings yet
- Kaye 1990Document5 pagesKaye 1990Bharat DedhiaNo ratings yet
- AlanervDocument3 pagesAlanervCen Janber CabrillosNo ratings yet
- Table of AntidotesDocument5 pagesTable of Antidotesangelotisbe1120No ratings yet
- L6-Steczina, Sonette BIOEN345 Lecture 20200415Document22 pagesL6-Steczina, Sonette BIOEN345 Lecture 20200415asdfghjklNo ratings yet
- Vitamins. Enzymatic Drugs and Their InhibitorsDocument2 pagesVitamins. Enzymatic Drugs and Their InhibitorsAsma AhmedNo ratings yet
- Histamina Metabolismo Ing.Document2 pagesHistamina Metabolismo Ing.Sócrates De AtenasNo ratings yet
- 4.1 Enzyme Chemistry Part 1Document7 pages4.1 Enzyme Chemistry Part 1Geraldine Marie SalvoNo ratings yet
- Roe 2002Document8 pagesRoe 2002JOHN JERALD VILLAMANCANo ratings yet
- Pharmac. TherDocument15 pagesPharmac. TherVeneta GizdakovaNo ratings yet
- The Pineal Gland and MelatoninDocument17 pagesThe Pineal Gland and Melatoninjheimis milaniNo ratings yet
- Protein and Amino Acids: Metabolism and AnalysisDocument35 pagesProtein and Amino Acids: Metabolism and AnalysisWindi MoseNo ratings yet
- Zinc and ThymulinDocument2 pagesZinc and ThymulinScottNo ratings yet
- Common Drugs and Their AntidotesDocument6 pagesCommon Drugs and Their AntidotesShun Reigh SumilangNo ratings yet
- Anemia-A SysthesisDocument88 pagesAnemia-A SysthesisMichelle San Miguel FeguroNo ratings yet
- 2000 Mechanisms of Vitamin Deficiency in Chronic Alcohol MisusersDocument6 pages2000 Mechanisms of Vitamin Deficiency in Chronic Alcohol MisusersMuhammad Sona KhanNo ratings yet
- A Rare Case of Acute Abdomen Secondary To Omental.283Document1 pageA Rare Case of Acute Abdomen Secondary To Omental.283prabowoaji12No ratings yet
- Lignan & LigninDocument27 pagesLignan & Ligninmuhammad fathiNo ratings yet
- Potassium-Sparing Diuretics: Drug Name Clinical Use Phacmacodynamics Toxicity Mineralocorticoid Receptor AntagonistDocument1 pagePotassium-Sparing Diuretics: Drug Name Clinical Use Phacmacodynamics Toxicity Mineralocorticoid Receptor AntagonistSalomeSibashviliNo ratings yet
- Methionine Metabolism in Heath and Cancer: A Nexus of Diet and Precision MedicienDocument13 pagesMethionine Metabolism in Heath and Cancer: A Nexus of Diet and Precision MediciendffNo ratings yet
- AntidoteDocument5 pagesAntidoteMaynard ArandaNo ratings yet
- AntidoteDocument8 pagesAntidotedeanelaylayNo ratings yet
- MLS 044 Clinical Bacteriology Session 10Document5 pagesMLS 044 Clinical Bacteriology Session 10JJ AngNo ratings yet
- Carbapenems PDFDocument18 pagesCarbapenems PDFElizabethHanganuNo ratings yet
- Early Family Life: Open-Versus Closed-Ended QuestionsDocument7 pagesEarly Family Life: Open-Versus Closed-Ended QuestionsRubie Ann TillorNo ratings yet
- Concept Map 1Document3 pagesConcept Map 1Rubie Ann TillorNo ratings yet
- HemorrhoidsDocument6 pagesHemorrhoidsRubie Ann TillorNo ratings yet
- VertigoDocument1 pageVertigoRubie Ann TillorNo ratings yet
- Both An Excess of Cortisol and DST Nonsuppression Have Been Reported For Many Years in Patients With Mood DisordersDocument12 pagesBoth An Excess of Cortisol and DST Nonsuppression Have Been Reported For Many Years in Patients With Mood DisordersRubie Ann TillorNo ratings yet
- Heart FailureDocument11 pagesHeart FailureRubie Ann TillorNo ratings yet
- Generalized Seizures: Absence Seizures. Absence Seizures, Previously Known As Petit MalDocument1 pageGeneralized Seizures: Absence Seizures. Absence Seizures, Previously Known As Petit MalRubie Ann TillorNo ratings yet
- Pathophysiology of Depression: Molecular Regulation of Melatonin Homeostasis - Current StatusDocument13 pagesPathophysiology of Depression: Molecular Regulation of Melatonin Homeostasis - Current StatusRubie Ann TillorNo ratings yet
- VertigoDocument1 pageVertigoRubie Ann TillorNo ratings yet
- StabismusDocument1 pageStabismusRubie Ann TillorNo ratings yet
- Diabetic RetinopathyDocument1 pageDiabetic RetinopathyRubie Ann TillorNo ratings yet
- Neonatal Sepsis Is A Type ofDocument1 pageNeonatal Sepsis Is A Type ofRubie Ann TillorNo ratings yet
- SepsisDocument1 pageSepsisRubie Ann TillorNo ratings yet
- Receptors Vision Vertebrates Camera Retina: Eyeball, Spheroidal Structure Containing SenseDocument1 pageReceptors Vision Vertebrates Camera Retina: Eyeball, Spheroidal Structure Containing SenseRubie Ann TillorNo ratings yet
- CC Rubie Ann G. Tillor: Chlorpromazin eDocument18 pagesCC Rubie Ann G. Tillor: Chlorpromazin eRubie Ann TillorNo ratings yet
- Receptors Vision Vertebrates Camera Retina: Eyeball, Spheroidal Structure Containing SenseDocument1 pageReceptors Vision Vertebrates Camera Retina: Eyeball, Spheroidal Structure Containing SenseRubie Ann TillorNo ratings yet
- FloatersDocument1 pageFloatersRubie Ann TillorNo ratings yet
- Receptors Vision Vertebrates Camera Retina: Eyeball, Spheroidal Structure Containing SenseDocument1 pageReceptors Vision Vertebrates Camera Retina: Eyeball, Spheroidal Structure Containing SenseRubie Ann TillorNo ratings yet
- CC Rubie Ann G. Tillor: IM: (Schizophrenia)Document11 pagesCC Rubie Ann G. Tillor: IM: (Schizophrenia)Rubie Ann TillorNo ratings yet
- Visceral Pain: Nausea, Vomiting, and Increased Heart Rate May Accompany Visceral PainDocument3 pagesVisceral Pain: Nausea, Vomiting, and Increased Heart Rate May Accompany Visceral PainRubie Ann TillorNo ratings yet
- Patient Health Questionnaire (Phq-9) : Date: NameDocument2 pagesPatient Health Questionnaire (Phq-9) : Date: NameRubie Ann TillorNo ratings yet
- Physical Examination of The HeartDocument8 pagesPhysical Examination of The HeartRubie Ann TillorNo ratings yet
- Oscillopsia Is The Illusion of Oscillation of The Visual Surroundings While Vertigo Refers To A Sense of Spinning or Other Motion That May Be PhysiologicalDocument2 pagesOscillopsia Is The Illusion of Oscillation of The Visual Surroundings While Vertigo Refers To A Sense of Spinning or Other Motion That May Be PhysiologicalRubie Ann TillorNo ratings yet
- Vital Signs: Results Interpretation: Pale ConjunctivaeDocument9 pagesVital Signs: Results Interpretation: Pale ConjunctivaeRubie Ann TillorNo ratings yet
- Receptors Vision Vertebrates Camera Retina: Eyeball, Spheroidal Structure Containing SenseDocument1 pageReceptors Vision Vertebrates Camera Retina: Eyeball, Spheroidal Structure Containing SenseRubie Ann TillorNo ratings yet
- Good Morning DoctorsDocument2 pagesGood Morning DoctorsRubie Ann TillorNo ratings yet
- JVP Measured at 3 CM Above The Sternal Angle, or 8 CM Above The Right Atrium, Is Considered Elevated or AbnormalDocument11 pagesJVP Measured at 3 CM Above The Sternal Angle, or 8 CM Above The Right Atrium, Is Considered Elevated or AbnormalRubie Ann TillorNo ratings yet
- SeqLab Exosap USB PDFDocument12 pagesSeqLab Exosap USB PDFYorladyNo ratings yet
- Biology A Level Handbook 2023Document34 pagesBiology A Level Handbook 2023uzair madanessNo ratings yet
- T Cell Flyer PDFDocument26 pagesT Cell Flyer PDFJohnny AtmanNo ratings yet
- Microbial NutritionDocument24 pagesMicrobial NutritionDeepak MudaraddiNo ratings yet
- Unraveling The Complexity of Liver Disease One Cell at A TimeDocument21 pagesUnraveling The Complexity of Liver Disease One Cell at A Timeguohong huNo ratings yet
- Soalan BK1 Finalyre15Document12 pagesSoalan BK1 Finalyre15hanafizaNo ratings yet
- Medchem of ANSDocument149 pagesMedchem of ANSeoeltt78No ratings yet
- Extremophiles 2004 PDFDocument149 pagesExtremophiles 2004 PDFChandraprasad S RajanganNo ratings yet
- Hemoglobin SigmaDocument3 pagesHemoglobin SigmascheekoNo ratings yet
- Systemic Response To Injury and MCQDocument3 pagesSystemic Response To Injury and MCQMalumo MisheckNo ratings yet
- 2nd - Part 3 - Lipid Structure and MetabolismDocument56 pages2nd - Part 3 - Lipid Structure and MetabolismAkbarWirawanNo ratings yet
- Link PL E-Katalog Dan Reguler - 2022 - Update 09112022Document28 pagesLink PL E-Katalog Dan Reguler - 2022 - Update 09112022Lince WijoyoNo ratings yet
- Harry Potter and The Prisoner of AzkabanDocument2 pagesHarry Potter and The Prisoner of Azkabanfrenzys nicie a. caburaoNo ratings yet
- Report Bio 2Document8 pagesReport Bio 2Hồng NhungNo ratings yet
- 102PDFDocument41 pages102PDFmayorca2310No ratings yet
- Bacterial Genetic SystemDocument13 pagesBacterial Genetic SystemKaiyama AkhtarNo ratings yet
- Minerals: By: Andrea Verencia Naftali Vita Zuhfatul MaulaDocument16 pagesMinerals: By: Andrea Verencia Naftali Vita Zuhfatul MaulaIdasari DewiNo ratings yet
- Kling 2017Document63 pagesKling 2017jainigNo ratings yet
- pETDuet 1 PDFDocument2 pagespETDuet 1 PDFesn_k100% (1)
- 2016 Life Science BioradDocument462 pages2016 Life Science BioradThị Sô PhiaNo ratings yet
- Pgi Chandigarh May 2010 EbookDocument49 pagesPgi Chandigarh May 2010 EbookJeetendra Singh100% (2)
- (Oklahoma Notes) Roger Thies Ph.D. (Auth.), Roger Thies Ph.D. (Eds.) - Physiology-Springer-Verlag New York (1995)Document287 pages(Oklahoma Notes) Roger Thies Ph.D. (Auth.), Roger Thies Ph.D. (Eds.) - Physiology-Springer-Verlag New York (1995)MaadaNo ratings yet
- Abiotic Stress Management QuizDocument2 pagesAbiotic Stress Management QuizLuke ShantiNo ratings yet
- Antifungal and Mycotoxin Detoxification Ability of Essential Oils - A ReviewDocument11 pagesAntifungal and Mycotoxin Detoxification Ability of Essential Oils - A ReviewsovalaxNo ratings yet
- 4c. Full ProceedingDocument626 pages4c. Full ProceedingRato Petani Bandeng SilamonNo ratings yet
- Purslane Portulaca Oleracea Seed Consumption and ADocument11 pagesPurslane Portulaca Oleracea Seed Consumption and Alucaste50No ratings yet
- Lecture 2 - Introductory BiochemistryDocument15 pagesLecture 2 - Introductory BiochemistryJana-Tae KerrNo ratings yet
- Biotechnology and Its ApplicationDocument13 pagesBiotechnology and Its ApplicationMaheswari RajnarayananNo ratings yet
- Name Designation Department Faculty E-Mail AddressDocument3 pagesName Designation Department Faculty E-Mail AddressMuhammad Adnan LaghariNo ratings yet