Professional Documents
Culture Documents
EEE 132 / ETE 132 Introduction To Materials and Chemistry Ideal Gas Equation Continued.
Uploaded by
MD. SHAEKH ZAHAB CHOWDHURY0 ratings0% found this document useful (0 votes)
11 views13 pages1) The document discusses the kinetic-molecular model of an ideal gas, which represents a gas as particles bouncing around in a closed container.
2) It describes the assumptions of the model, including that gas particles are small, in constant motion, and collide elastically with the container walls.
3) The document explains how molecular collisions with the container walls exert pressure on the walls, and how this pressure can be related to temperature based on the kinetic energy of the gas molecules.
Original Description:
Original Title
EEE13201- Lecture 6
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this Document1) The document discusses the kinetic-molecular model of an ideal gas, which represents a gas as particles bouncing around in a closed container.
2) It describes the assumptions of the model, including that gas particles are small, in constant motion, and collide elastically with the container walls.
3) The document explains how molecular collisions with the container walls exert pressure on the walls, and how this pressure can be related to temperature based on the kinetic energy of the gas molecules.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
11 views13 pagesEEE 132 / ETE 132 Introduction To Materials and Chemistry Ideal Gas Equation Continued.
Uploaded by
MD. SHAEKH ZAHAB CHOWDHURY1) The document discusses the kinetic-molecular model of an ideal gas, which represents a gas as particles bouncing around in a closed container.
2) It describes the assumptions of the model, including that gas particles are small, in constant motion, and collide elastically with the container walls.
3) The document explains how molecular collisions with the container walls exert pressure on the walls, and how this pressure can be related to temperature based on the kinetic energy of the gas molecules.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 13
EEE 132 / ETE 132 Introduction
to Materials and Chemistry
Lecture 6
Ideal Gas Equation Continued..
Kazi Naziba Tahsin
Lecturer
School of Engineering and Computer Science
Independent University, Bangladesh
Kinetic-Molecular Model of an Ideal Gas
• The goal of any molecular theory of matter is to understand the
macroscopic properties of matter in terms of its atomic or molecular
structure and behavior.
• Once we have this understanding, we can design materials to have
specific desired properties. Theories have led to the development of
high-strength steels, semiconductor materials for electronic devices,
and countless other materials essential to contemporary technology.
In this and the following sections we will consider a simple molecular
model of an ideal gas. This kinetic-molecular model represents the gas
as a large number of particles bouncing around in a closed container.
In this section we use the kinetic-molecular model to understand how
the ideal-gas equation of state.
• Here are the assumptions of our model:
• 1. A container with volume V contains a very large number N of
identical molecules, each with mass m.
• 2. The molecules behave as point particles that are small compared to
the size of the container and to the average distance between
molecules.
• 3. The molecules are in constant motion. Each molecule collides
occasionally with a wall of the container. These collisions are perfectly
elastic.
• 4. The container walls are rigid and infinitely massive and do not move.
Collisions and Gas Pressure
• During collisions the molecules exert forces on the walls of the
container; this is the origin of the pressure that the gas exerts.
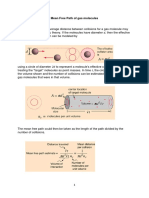
In a typical collision (Fig. below) the velocity component
parallel to the wall is unchanged, and the component
perpendicular to the wall reverses direction but does not
change in magnitude.
• Our program is first to determine the number of collisions that
occur per unit time for a certain area A of wall. Then we find
the total momentum change associated with these collisions
and the force needed to cause this momentum change. From
this we can determine the pressure, which is force per unit
area, and compare the result to the ideal-gas equation. We’ll
find a direct connection between the temperature of the gas
and the kinetic energy of the gas molecules
• To begin, we will assume that all molecules in the gas have the same
magnitude of x-velocity vx, This isn’t right, but making this temporary
assumption helps to clarify the basic ideas. We will show later that this
assumption isn’t really necessary.
Pressure and Molecular Kinetic Energies
Molecular Speeds
Problems
• Example 18.6 Molecular kinetic energy and vrms
• What is the average translational kinetic energy of an ideal-gas
molecule at (b) What is the total random translational kinetic energy
of the molecules in 1 mole of this gas? (c) What is the root-mean-
square speed of oxygen molecules at this temperature?
• Calculating rms and average speeds
• Five gas molecules chosen at random are found to have speeds of
500, 600, 700, 800, and What is the rms speed? What is the average
speed?
Read pg 604 – 605 (university physics)
• Calculating mean free path
You might also like
- Physical ChenistryDocument146 pagesPhysical ChenistrychemasimNo ratings yet
- Investigatory Project PhyDocument11 pagesInvestigatory Project Phylavanya rajaNo ratings yet
- Modeling Molecular Structures With Hyperchem: Computational Chemistry LaboratoryDocument13 pagesModeling Molecular Structures With Hyperchem: Computational Chemistry LaboratorySwarali KamathNo ratings yet
- (The Berkeley Review) The Berkeley Review MCAT GenDocument324 pages(The Berkeley Review) The Berkeley Review MCAT GenSara El100% (3)
- Gas Laws: Sagana National High SchoolDocument17 pagesGas Laws: Sagana National High SchoolHelma Jabello AriolaNo ratings yet
- Physical Chemistry-I - Dr. SMDocument234 pagesPhysical Chemistry-I - Dr. SMrohitkm298No ratings yet
- Kinetic Theory of GasesDocument43 pagesKinetic Theory of GasesAbdur RahmanNo ratings yet
- 9.5 The Kinetic-Molecular Theory - Chemistry 2e - OpenStaxDocument6 pages9.5 The Kinetic-Molecular Theory - Chemistry 2e - OpenStaxBillyNo ratings yet
- Lecture 1Document53 pagesLecture 1Virat BhangeNo ratings yet
- Molecular Dynamics Simulations: Dr.M.Chandra SekharDocument24 pagesMolecular Dynamics Simulations: Dr.M.Chandra SekharchandraloveNo ratings yet
- Lecture 6 - Fall 2023-24Document11 pagesLecture 6 - Fall 2023-24rtasin9No ratings yet
- Gas Laws: Sagana National High SchoolDocument17 pagesGas Laws: Sagana National High SchoolCHRYSTER TASHANA DANNE GAMIAONo ratings yet
- Kinetic-Molecular Theory: Ideal Gas Equation PV NRTDocument9 pagesKinetic-Molecular Theory: Ideal Gas Equation PV NRTVidhuPandey100% (1)
- Kinetic TheoryDocument20 pagesKinetic Theorykishorkumarn8212No ratings yet
- Kinetic TheoryDocument26 pagesKinetic TheoryDelano PeteNo ratings yet
- Kinetic Theory of GasDocument5 pagesKinetic Theory of Gasbenazeerbeevi9No ratings yet
- 02 - Kinetic Gas Equation Derivation - RMS Velocity FormulaDocument4 pages02 - Kinetic Gas Equation Derivation - RMS Velocity FormulaDeepakNo ratings yet
- Kinetic Molecular TheoryDocument4 pagesKinetic Molecular TheoryJOHN ROLIE MAMELOCONo ratings yet
- Chem Entry #4Document67 pagesChem Entry #4Vivialyn YumulNo ratings yet
- High Temperature Gas Dynamics - Synopsis - Himanshu BanaitDocument53 pagesHigh Temperature Gas Dynamics - Synopsis - Himanshu BanaitHimanshu BanaitNo ratings yet
- Kinetic TheoryDocument7 pagesKinetic TheoryngvanduysnNo ratings yet
- Mean Free PathDocument9 pagesMean Free PathRestiAyuNo ratings yet
- Module-1: Tensor Algebra: Lecture-1: Introduction To Continuum MechanicsDocument3 pagesModule-1: Tensor Algebra: Lecture-1: Introduction To Continuum MechanicsroysiddarthNo ratings yet
- Kinetic Molecular Theory of Gases WorksheetDocument2 pagesKinetic Molecular Theory of Gases WorksheetJudi TurkNo ratings yet
- Kinetic Theory of GasesDocument30 pagesKinetic Theory of GasesAyushNo ratings yet
- BI 2 - Kinetic Molecular TheoryDocument23 pagesBI 2 - Kinetic Molecular TheoryfihiNo ratings yet
- The Model: The Volume Occupied by The Molecules of The Gas Is Negligible Compared To The Volume of The Gas ItselfDocument1 pageThe Model: The Volume Occupied by The Molecules of The Gas Is Negligible Compared To The Volume of The Gas ItselfSameer Singh PatelNo ratings yet
- Lecture 1Document12 pagesLecture 1Sima KumariNo ratings yet
- RevModPhys 75 121Document60 pagesRevModPhys 75 121Furkan OkNo ratings yet
- Gas Dynamics and Jet Propulsion For MG University s6 Mechanical StudentsDocument314 pagesGas Dynamics and Jet Propulsion For MG University s6 Mechanical StudentsBASIL BENNY100% (1)
- Buku Termo - RemovedDocument206 pagesBuku Termo - RemovedRika YussafitriNo ratings yet
- 1.1 Continuum Theory: KnownDocument2 pages1.1 Continuum Theory: KnownJason OliverNo ratings yet
- ch05 PDFDocument8 pagesch05 PDFFAIQNo ratings yet
- Molecular ModellingDocument15 pagesMolecular Modelling2k22cscys2213057No ratings yet
- Physics Kinetic Theory LessonDocument24 pagesPhysics Kinetic Theory LessonRufat IsmayilovNo ratings yet
- Molecular Vibrations: The Theory of Infrared and Raman Vibrational SpectraFrom EverandMolecular Vibrations: The Theory of Infrared and Raman Vibrational SpectraNo ratings yet
- 13.01 Kinetic Molecular TheoryDocument2 pages13.01 Kinetic Molecular TheoryReshma GuptaNo ratings yet
- The Kinetic Theory of GasesDocument22 pagesThe Kinetic Theory of GasesMegis HefrindhaNo ratings yet
- B03 - Mean Free PathDocument2 pagesB03 - Mean Free PathMohamed ELMOUHINNINo ratings yet
- Thermal Physics - 4Document1 pageThermal Physics - 4AliiAmiirNo ratings yet
- Comp Chem Lecture 1Document27 pagesComp Chem Lecture 1mvikosiphosethu2407No ratings yet
- Kinetic Molecular ThasdeoryDocument1 pageKinetic Molecular ThasdeoryVirgilio SedantoNo ratings yet
- Introduction to Electromagnetic EngineeringFrom EverandIntroduction to Electromagnetic EngineeringRating: 5 out of 5 stars5/5 (1)
- Kinetic Molecular Theory Group 3Document22 pagesKinetic Molecular Theory Group 3Carl Ritchie TempleNo ratings yet
- Size-Dependent Elastic Properties of A Single-Walled Carbon Nanotube Via A Molecular Mechanics Model Chang and Gao 2003 PDFDocument16 pagesSize-Dependent Elastic Properties of A Single-Walled Carbon Nanotube Via A Molecular Mechanics Model Chang and Gao 2003 PDFKishore Guru-MENo ratings yet
- Gaseous & Liquid StatesDocument51 pagesGaseous & Liquid Statesakbar azamNo ratings yet
- Lesson 2Document17 pagesLesson 2Vamsi Krishna ChaitanyaNo ratings yet
- CHAPTER 1 Introduction 2010 Introduction To Continuum Mechanics Fourth EditionDocument2 pagesCHAPTER 1 Introduction 2010 Introduction To Continuum Mechanics Fourth EditionJoão Carlos QueirozNo ratings yet
- Mean Free PathsDocument3 pagesMean Free PathsAISHEE DIBANo ratings yet
- Intermolecular Forces and Its Applications: For General Chemistry 2/grade 12 (STEM) Quarter 3/week 1.a-DDocument19 pagesIntermolecular Forces and Its Applications: For General Chemistry 2/grade 12 (STEM) Quarter 3/week 1.a-DSherwin Jay PalaspasNo ratings yet
- The Gaseous State: The Commonwealth and International Library: Chemistry DivisionFrom EverandThe Gaseous State: The Commonwealth and International Library: Chemistry DivisionNo ratings yet
- Kinetic Molecular Model of A GasDocument6 pagesKinetic Molecular Model of A GasUsman GhaniNo ratings yet
- Experiment 4Document5 pagesExperiment 4patelp4026No ratings yet
- Kinetic Theory of GasesDocument8 pagesKinetic Theory of GasesLeslie JusufNo ratings yet
- Review6 9Document4 pagesReview6 9MinTekenNo ratings yet
- Unit 1: Gas LawDocument17 pagesUnit 1: Gas LawDennise AguilarNo ratings yet
- Chapter 12 Kinetic Theory-1Document27 pagesChapter 12 Kinetic Theory-1Anil KumarNo ratings yet
- Computer in Medicinal ChemistryDocument48 pagesComputer in Medicinal ChemistryFarmasi SMKNegeri1SambiNo ratings yet
- PHY11101 - Periodic MotionDocument5 pagesPHY11101 - Periodic MotionMD. SHAEKH ZAHAB CHOWDHURYNo ratings yet
- PHY11101 - Mechanical WavesDocument3 pagesPHY11101 - Mechanical WavesMD. SHAEKH ZAHAB CHOWDHURYNo ratings yet
- Eee/Ete 132 Introduction To Materials and Chemistry: Thermodynamics and Enthalpy For EngineersDocument32 pagesEee/Ete 132 Introduction To Materials and Chemistry: Thermodynamics and Enthalpy For EngineersMD. SHAEKH ZAHAB CHOWDHURYNo ratings yet
- EEE 132 / ETE 132 Introduction To Materials and ChemistryDocument29 pagesEEE 132 / ETE 132 Introduction To Materials and ChemistryMD. SHAEKH ZAHAB CHOWDHURYNo ratings yet
- Modified Motor PDFDocument11 pagesModified Motor PDFMD. SHAEKH ZAHAB CHOWDHURYNo ratings yet
- MO UNIT - I PPT NotesDocument81 pagesMO UNIT - I PPT NotesKrishnan DhanasekaranNo ratings yet
- Set6ans 10Document4 pagesSet6ans 10Natália FerreiraNo ratings yet
- SuppEx Solution 2B EDocument133 pagesSuppEx Solution 2B EElva liNo ratings yet
- KIL 1007 Physical Chemistry Laboratory: Dr. Tan Chee KeongDocument8 pagesKIL 1007 Physical Chemistry Laboratory: Dr. Tan Chee KeongSafayet AzizNo ratings yet
- Chemistry 112 Dye Kinetics Laboratory 2011Document4 pagesChemistry 112 Dye Kinetics Laboratory 2011Lavenia Alou MagnoNo ratings yet
- Stoichiometry CPPDocument4 pagesStoichiometry CPPkalmee ram MeenaNo ratings yet
- Heat and Material Balances Around Rectifying ColumnDocument5 pagesHeat and Material Balances Around Rectifying ColumnDaniel ObasNo ratings yet
- Experiment 2 GRANDA JOHNDocument7 pagesExperiment 2 GRANDA JOHNKENT BENEDICT PERALESNo ratings yet
- Metal Ions in Biological SystemsDocument10 pagesMetal Ions in Biological SystemsRiyani LangiNo ratings yet
- Product Summaries Mining Application v09Document1 pageProduct Summaries Mining Application v09Ari WijayaNo ratings yet
- Acs Iecr 0c04760Document23 pagesAcs Iecr 0c04760Jose Manuel Serrano Del RioNo ratings yet
- Gopi Krishna (Autosaved)Document11 pagesGopi Krishna (Autosaved)Gopi KrishNo ratings yet
- Hazardous Waste ManagementDocument13 pagesHazardous Waste ManagementdaabgchiNo ratings yet
- Aman Soni (Corrosion Assessment) - 1 PDFDocument32 pagesAman Soni (Corrosion Assessment) - 1 PDFAmAn SoNiNo ratings yet
- A Review of Heat Transfer Enhancement Using Twisted Tape With and Without PerforationDocument9 pagesA Review of Heat Transfer Enhancement Using Twisted Tape With and Without PerforationIJIERT-International Journal of Innovations in Engineering Research and TechnologyNo ratings yet
- Alcohols and Modern Analytical Techniques HWDocument11 pagesAlcohols and Modern Analytical Techniques HWcocoayisaNo ratings yet
- Enzymes Lab ReportDocument7 pagesEnzymes Lab ReportMemorie BrownNo ratings yet
- Activity No 3 Chemical NomenclatureDocument9 pagesActivity No 3 Chemical NomenclatureVaron Soriano SulitNo ratings yet
- Condensate Water Polishing SystemDocument257 pagesCondensate Water Polishing SystemHamza RaoNo ratings yet
- Calculating The Sodium Chloride Equivalent of A SubstanceDocument8 pagesCalculating The Sodium Chloride Equivalent of A SubstanceLorenzOrminitaNo ratings yet
- AS Chemistry Answer Sheet 02 AnsDocument10 pagesAS Chemistry Answer Sheet 02 Ansthegreatwardini0% (1)
- Polymer Testing: W. Stark, M. JaunichDocument7 pagesPolymer Testing: W. Stark, M. JaunichJohnSmithNo ratings yet
- Infographic About Reaction RatesDocument2 pagesInfographic About Reaction Ratesnick.chang14No ratings yet
- Case Study Numerical Simulation of Surfactant Flooding in Low Permeability Oil Filed PDFDocument11 pagesCase Study Numerical Simulation of Surfactant Flooding in Low Permeability Oil Filed PDFDavid LópezNo ratings yet
- Annulus Gas Monitoring System6Document9 pagesAnnulus Gas Monitoring System6Narayan SutharNo ratings yet
- Class10 IITJEEDocument17 pagesClass10 IITJEEMayyank Garg75% (4)
- Grade 8 Science - Q3 - Trends in The Periodic TableDocument6 pagesGrade 8 Science - Q3 - Trends in The Periodic TableKeziah Costelo50% (2)
- Introduction To PH PDFDocument3 pagesIntroduction To PH PDFRichard ObinnaNo ratings yet
- A Calibration Model For Screen Caged Peltier ThermocoupleDocument160 pagesA Calibration Model For Screen Caged Peltier Thermocoupleandressaoliveira2301No ratings yet
- MIDTERM Enzymology 1Document20 pagesMIDTERM Enzymology 1Sammy MñNo ratings yet