12/30/2020 Destructive distillation - Wikipedia
Destructive distillation
Destructive distillation is a chemical process in which decomposition of unprocessed material is achieved by heating it to a high temperature;
the term generally applies to processing of organic material in the absence of air or in the presence of limited amounts of oxygen or other reagents,
catalysts, or solvents, such as steam or phenols. It is an application of pyrolysis. The process breaks up or 'cracks' large molecules. Coke, coal gas,
gas carbon, coal tar, ammonia liquor, and coal oil are examples of commercial products historically produced by the destructive distillation of coal.
Destructive distillation of any particular inorganic feedstock produces only a small range of products
as a rule, but destructive distillation of organic materials commonly produces very many
compounds, often hundreds, although not all products of any particular process are of commercial
importance. The distillate are generally lower molecular weight. Some fractions however polymerise
or condense small molecules into larger molecules, including heat-stable tarry substances and chars.
Cracking feedstocks into liquid and volatile compounds, and polymerising, or the forming of chars
and solids, may both occur in the same process, and any class of the products might be of
commercial interest.
Currently the major industrial application of destructive distillation is to coal.[1][2]
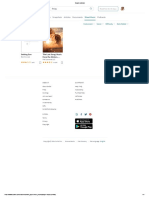
Historically the process of destructive distillation and other forms of pyrolysis led to the discovery of Many early experiments used retorts for
many chemical compounds or elucidation of their structures before contemporary organic chemists destructive distillation.
had developed the processes to synthesise or specifically investigate the parent molecules. It was
especially in the early days that investigation of the products of destructive distillation, like those of
other destructive processes, played parts in enabling chemists to deduce the chemical nature of many natural materials.[3] Well known examples
include the deduction of the structures of pyranoses and furanoses.[4]
Contents
Process
Applications
See also
References
External links
Process
The process of pyrolysis can be conducted in a distillation apparatus (retort) to form the volatile products for collection. The mass of the product will
represent only a part of the mass of the feedstock, because much of the material remains as char, ash, and non-volatile tars. In contrast, combustion
consumes most of the organic matter, and the net weight of the products amount to roughly the same mass as the fuel and oxidant consumed.
Destructive distillation and related processes are in effect the modern industrial descendants of traditional charcoal burning crafts. As such they are
of industrial significance in many regions, such as Scandinavia. The modern processes are sophisticated and require careful engineering to produce
the most valuable possible products from the available feedstocks.[5][6]
Applications
Destructive distillation of wood produces hundreds of compounds including tar, terpenes, turpentine and methanol together with a solid residue
of charcoal.[7][8]
Destructive distillation of a tonne of coal can produce 700 kg of coke, 100 liters of liquor ammonia, 50 liters of coal tar and 400 m3 of coal gas.
Destructive distillation is an increasingly promising method for recycling monomers derived from waste polymers.
Destructive distillation of natural rubber resulted in the discovery of Isoprene which led to the creation of synthetic rubbers such as neoprene.[9]
See also
Dry distillation
Pyrolysis
Thermolysis
Cracking (chemistry)
References
1. Lunge, George (1887). Coal-tar and ammonia (https://archive.org/details/coaltarandammon00lunggoog). Gurney and Jackson.
2. Speight, James G. (2010). The Refinery of the Future. William Andrew. ISBN 978-0-8155-2041-2.
3. Schorlemmer, Carl; Smithells, Arthur (1894). The rise and development of organic chemistry (https://archive.org/details/risedevelopmento00sch
orich). Macmillan.
4. I.L. Finar Organic Chemistry vol 1 ( 4th.ed.) Longmans 1963 plus I.L. Finar Organic Chemistry vol 2 ( 3rd.ed.) Longmans Green & Co. 1964 May
be downlaoded from: https://archive.org/details/OrganicChemistryVol1 plus https://archive.org/details/OrganicChemistryVol2
5. Bates, John S.; Distillation of hardwoods in Canada; Pub: Ottawa, F. A. Acland, 1922. May be downloaded from: [1] (https://archive.org/details/di
stillationofha00baterich)
https://en.wikipedia.org/wiki/Destructive_distillation 1/2
�12/30/2020 Destructive distillation - Wikipedia
6. Klar, Max; Rule, Alexander; The technology of wood distillation, with special reference to the methods of obtaining the intermediate and finished
products from the primary distillate; Pub: London Chapman & Hall 1925. May be downloaded from: [2] (https://archive.org/details/technologyofw
ood00klaruoft)
7. Loos, Hermann A.; A Study on Colophony Resin; Columbia University 1900. May be downloaded from: [3] (https://archive.org/details/astudycolo
phony00loosgoog)
8. DUMESNY, P. & NOYER J.; Wood products, distillates and extracts; Pub: Scott, Greenwood & Son, 1908. May be downloaded from: [4] (https://
archive.org/details/woodproductsdist00dumeuoft)
9. Greville Williams, C. (1860). "On Isoprene and Caoutchine". Proceedings of the Royal Society of London. 10: 516–519. JSTOR 111688 (https://
www.jstor.org/stable/111688).
External links
What is destructive distillation ? (http://www.wisegeek.com/what-is-destructive-distillation.htm)
Retrieved from "https://en.wikipedia.org/w/index.php?title=Destructive_distillation&oldid=977933488"
This page was last edited on 11 September 2020, at 21:13 (UTC).
Text is available under the Creative Commons Attribution-ShareAlike License; additional terms may apply. By using this site, you agree to the Terms of Use and Privacy Policy.
Wikipedia® is a registered trademark of the Wikimedia Foundation, Inc., a non-profit organization.
https://en.wikipedia.org/wiki/Destructive_distillation 2/2